Advertisements
Advertisements
प्रश्न
Write balanced equation for the preparation of nitric acid from potassium nitrate.
उत्तर
\[\ce{KNO3 + H2SO4 ->[200°] KHSO4 + HNO3}\]
APPEARS IN
संबंधित प्रश्न
Write a balanced chemical equation for the following:
Action of cold and dilute Nitric acid on Copper
Explain the following:
Concentrated nitric add appears yellow when it is left standing in a glass bottle.
What is the purpose of Conc. H2SO4 in the above preparation?
Explain with the help of a balanced equation, the brown ring test for nitric acid.
What do you observe when?
Scrap zinc is heated with conc. HNO3?
Write the equation for the reaction:
Aluminum, Nitride and Water.
Write a balanced equation for following :
Reaction of ammonia with nitric acid
The figure given below illustrates the apparatus used in the laboratory preparation of nitric acid.
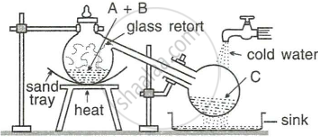
- Name A (a liquid), B (a solid), and C (a liquid). (Do not give the formulae).
- Write an equation to show how nitric acid undergoes decomposition.
- Write the equation for the reaction in which copper is oxidised by concentrated nitric acid.
From the following list of substances, choose one substance in the case which matches the description given below:
Ammonium nitrate, calcium hydrogen carbonate, copper carbonate, lead nitrate, potassium nitrate, sodium carbonate, sodium hydrogen carbonate, zinc carbonate.
A Substance that gives off only oxygen when heated.
Give a balanced equation for the following reaction:
Copper reacts with concentrated nitric acid.
