Advertisements
Advertisements
प्रश्न
Write a chemical equation to show the process of corrosion of iron.
उत्तर
In corrosion, oxidation of iron takes place.
Fe (s) → Fe2+ (aq) + 2e−
Iron supplies electrons at the edge of the droplet to reduce oxygen from the air.
O2 (g) + 2H2O (l) + 4e− → 4OH− (aq)
Within the droplet, the hydroxide ions react with the iron(II) ions and iron(II) hydroxide is precipitated.
Fe2+ (aq) + 2OH− (aq) → Fe(OH)2 (s)
Rust is then quickly produced by the oxidation of the precipitate.
4Fe(OH)2 (s) + O2 (g) → 2Fe2O3 • H2O (s) + 2H2O (l)
APPEARS IN
संबंधित प्रश्न
Explain the terms Corrosion
Which metals do not corrode easily?
What is meant by galvanisation? Why is it done?
Name the metal which is used for galvanising iron.
In one method of rust prevention, the iron is not coated with anything. Which is this method?
Fill in the following blank with suitable word:
The corrosion of iron is called ................
Fill in the following blanks with suitable words:
Tiffin boxes are electroplated with .............. but car bumpers are electroplated with ............... to protect them from rusting.
Corrosion can be an advantage in some case.Explain ?
Observe the following picture a write down the chemical reaction with the explanation.

Write scientific reasons.
Lemon or tamarind is used for cleaning copper vessels turned greenish.
Identify the process shown in the diagram and explain it in short
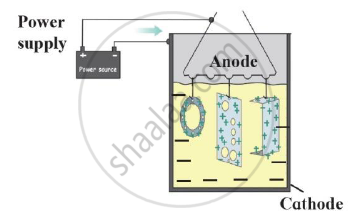
Give a reason why rust turns moist red litmus blue.
When one of the metals in an alloy is mercury the alloy is called _______.
Write a molecular formula for rust.
Give preventive methods by giving examples of corrosion?
Which among the following alloys contain mercury as one of its constituents?
The iron pillar near the Qutub Minar in Delhi is famous for the following facts. Which of these facts is responsible for its long stability?
Give an example of a chemical reaction for each of the following situations:
Sound is produced
In ______ process a layer of molten tin is deposited on metals.
