Advertisements
Advertisements
प्रश्न
Write the main product in the following reaction:
\[\ce{2CH3COCl + (CH3)2Cd ->}\]
उत्तर
\[\ce{2CH3COCl + (CH3)2Cd -> \underset{Propanone}{2CH3COCH3} + \underset{Cadmium Chloride}{CdCl2}}\]
संबंधित प्रश्न
Write the structure of the product of the following reaction:
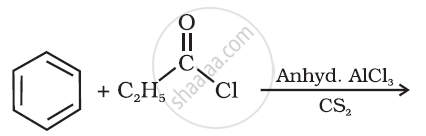
How will you bring about the following conversion in not more than two steps?
Benzene to m-Nitroacetophenone
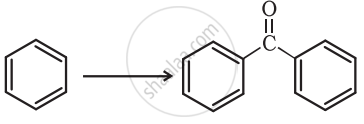
How will you bring about the following conversion in not more than two steps?
Benzaldehyde to Benzophenone
Describe the following:
Acetylation
Complete the synthesis by giving missing starting material, reagent or product.
Addition of water to alkynes occurs in acidic medium and in the presence of \[\ce{Hg^{2+}}\] ions as a catalyst. Which of the following products will be formed on addition of water to but-1-yne under these conditions.
Which of the following will not give the iodo form test?
Which of the following does not give iodo from test?
Which of the following compounds when treated with dibenzylcadmium yields benzyl methyl ketone?
An organic compound (X) with molecular formula C9H10O gives positive 2, 4-DNP and Tollen's test It undergoes Cannizarro reaction and on vigorous oxidation, it gives 1, 4-Benzenedicarboxylic acid. Compound (X) is:
