Advertisements
Advertisements
प्रश्न
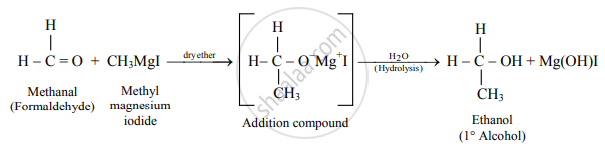
Write the preparation of ethanol from methyl magnesium iodide.
उत्तर
Methyl magnesium iodide reacts with formaldehyde to form an adduct which on hydrolysis with dilute acid gives ethanol.
APPEARS IN
संबंधित प्रश्न
Anisole on heating with concerntrated HI gives _________.
What is the action of atmospheric oxygen on ethers?
Write the reaction between ethanol and acetic anhydride.
How will you prepare diethyl ether by dehydration of alcohol?
What are the limitations to prepare ether by this method?
In the following reaction:
\[\ce{Ether ->[Hot HI] A + B + H2O}\],
If A and B are identical, the ether is ____________.
For the preparation of mixed ethers by Williamson synthesis, which of the following combination will give the best yield?
Action of hydrogen iodide on anisole gives ______.
____________ on heating with excess of conc. HI gives two moles of ethyl iodide.
Which of the following statements is INCORRECT for ethers?
A mixed ether on hydrolysis gives ____________.
To prepare ethanol from methyl magnesium bromide, the other reagent required is ____________.
When methoxy benzene reacts with HI at room temperature the products formed are ______.
What are the products of the following reaction?
\[\ce{(CH3)3C - O - CH3 + HI ->[cold] ?}\]
Which of the following compounds does NOT contain \[\begin{array}{cc}\backslash\phantom{.......}\\\ce{C = O}\\
/\phantom{.......}\\\end{array}\] group?
Ether is obtained from ethyl alcohol in presence of H2SO4 at _______.
The compound which is not formed when a mixture of n-butyl bromide and ethyl bromide treated with sodium metal in the presence of dry ether is ______.
Tert-butyl methyl ether on treatment with hydrogen iodide in cold gives ______.
Formation of diethyl ether from ethanol is based oh a ______.
Ethers when dissolved in cold concentrated sulphuric acid forms ______.
Why ethers possess a small net dipole moment?
