Advertisements
Advertisements
प्रश्न
Write the reactions of D-glucose which can’t be explained by its open-chain structure. How can cyclic structure of glucose explain these reactions?
उत्तर
Following reactions and facts could not be explained by open-chain structures of glucose.
• Despite having the aldehyde group, glucose does not give 2, 4 − DNP test, Schiff's test and it does not form the hydrogen sulphide addition product with \[\ce{NaHSO3}\].
• The pentaacetate of glucose does not react with hydroxylamine indicating the absence of a free −CHO group.
It was proposed that one of the −OH groups may add to the −CHO group and form a cyclic hemiacetal structure. It was found that glucose forms a six-membered ring in which −OH at C − 5 is involved in a ring formation.
APPEARS IN
संबंधित प्रश्न
Enumerate the reactions of D-glucose which cannot be explained by its open chain structure.
Acetylation of glucose yields ____________.
Which of the following statements is incorrect regarding glucose?
Glucose does not react with ____________.
Which is the least stable form of glucose?
The α-D glucose and β-D glucose differ from each other due to difference in carbon atom with respect to its ____________.
Three structures are given below in which two glucose units are linked. Which of these linkages between glucose units are between C1 and C4 and which linkages are between C1 and C6?
| (I) |  |
| (II) | 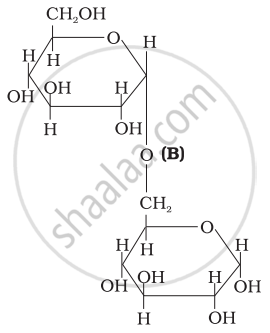 |
| (III) | 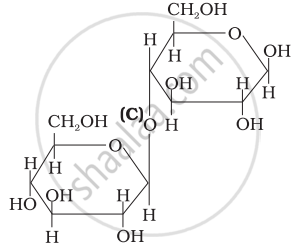 |
Assertion: D (+) – Glucose is dextrorotatory in nature.
Reason: ‘D’ represents its dextrorotatory nature.
On the basis of which evidences D-glucose was assigned the following structure?
\[\begin{array}{cc}
\ce{CHO}\\
|\phantom{....}\\
\phantom{..}\ce{(CHOH)4}\\
|\phantom{....}\\
\phantom{..}\ce{CH2OH}
\end{array}\]
Match List - I with List - II.
| List I | List II | ||
| (A) | Glucose + HI | (I) | Gluconic acid |
| (B) | Glucose + Br2 water | (II) | Glucose pentacetate |
| (C) | Glucose + acetic anhydride | (III) | Saccharic acid |
| (D) | Glucose + HNO3 | (IV) | Hexane |
Choose the correct answer from the options given below:
