Advertisements
Advertisements
प्रश्न
Write the structure of the product of the following reaction:
\[\ce{H3C - C ≡ C - H ->[Hg^{2+}, H2SO4]}\]
उत्तर
\[\begin{array}{cc}
\phantom{..............................}\ce{OH}\phantom{..................}\ce{O}\\
\phantom{..............................}|\phantom{.....................}||\phantom{}\\
\ce{H3C - \underset{Propyne}{C ≡ C} - H ->[Hg^{2+}, H2SO4] [CH3 - C = CH2] -> CH3 \underset{Propanone}{- C -} CH3}
\end{array}\]
APPEARS IN
संबंधित प्रश्न
Complete the synthesis by giving missing starting material, reagent or product.
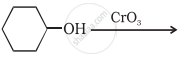
Write a note on ‘aldol condensation’.
What is the action of phenyl hydrazine on propanone?
What is the action of Sodium bisulphite on propanone?
Write chemical equation for the following :
Rosenmund reduction
What is the action of following reagents on ethanoic acid?
P2O5/heat
Draw structure of salicylaldehyde.
In the formation of an aldol, α-carbon atom of one aldehyde molecule attaches to ____________ of another aldehyde molecule.
Catalyst used in Rosenmund reduction is ____________.
Which of the following is called as mandelonitrile?
Which of the following represents Etard's reaction?
The reaction in which methyl group on benzene ring is converted to aldehydic group is called ______.
Identify product A in the following reaction:
\[\ce{Acetyl chloride + Dibenzylcadmium -> A}\]
Given below are two statements labelled as Assertion (A) and Reason (R).
Assertion (A): SO2 is reducing while TeO2 is an oxidising agent.
Reason (R): Reducing property of dioxide decreases from SO2 to TeO2.
Select the most appropriate answer from the options given below:
Give the reactions involved in the Etard's reaction.
Two isomers 'A' and 'B' with molecular formula C4H8 give different products on oxidation with KMnO4 in acidic medium. Isomer 'A' on reaction with KMnO4/H+ results in the effervescence of gas and gives ketone. The compound 'A' is:
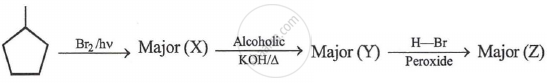
Z is:
How butanone is prepared from alcohol?
Write preparation of propanone by using ethanoyl chloride and dimethyl cadmium.
