Advertisements
Advertisements
Question
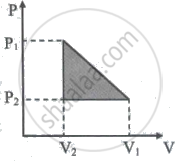
10 One mole of a van der Waals' gas obeying the equation `("P" + "a"/"V"^2)`(V - b) = RT undergoes the quasi-static cyclic process which is shown in the P-V diagram. The net heat absorbed by the gas in this process is ______
Options
`1/2("P"_1 - "P"_2)("V"_1 - "V"_2)`
`1/2("P"_1 + "P"_2)("V"_1 - "V"_2)`
`1/2("P"_1 + "a"/"V"_1^2 - "P"_2 - "a"/"V"_2^2)("V"_1 - "V"_2)`
`1/2("P"_1 + "a"/"V"_1^2 + "P"_2 + "a"/"V"_2^2)("V"_1 - "V"_2)`
Solution
10 One mole of a van der Waals' gas obeying the equation `("P" + "a"/"V"^2)`(V - b) = RT undergoes the quasi-static cyclic process which is shown in the P-V diagram. The net heat absorbed by the gas in this process is `underline(1/2("P"_1 - "P"_2)("V"_1 - "V"_2))`.
Explanation:
For cyclic process, beat absorbed
= work done
= Area = `1/2("P"_1 - "P"_2)("V"_1 - "V"_2)`
