Advertisements
Advertisements
Question
A student performs the following experiment in his school laboratory.
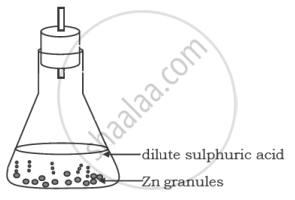
List two observations to justify that in this experiment a chemical change has taken place.
Solution
In the experiment where zinc (Zn) granules react with sulfuric acid (H2SO4), two observations that justify a chemical change are:
-
Evolution of Gas: When zinc reacts with sulfuric acid, hydrogen gas (H2) is released. This can be observed as bubbling or effervescence around the zinc granules. The production of a gas is a key indicator of a chemical change.
-
Heat Generation: The reaction between zinc and sulfuric acid is exothermic, meaning it releases heat. The student would notice a temperature increase in the reaction mixture, further indicating a chemical change is occurring.
These observations confirm that a chemical reaction has taken place as new products are formed (zinc sulfate and hydrogen gas).
