Advertisements
Advertisements
Question
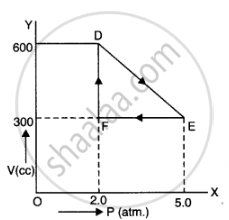
A thermodynamic system is taken from an original state to an intermediate state by the linear process shown in Figure

Its volume is then reduced to the original value from E to F by an isobaric process. Calculate the total work done by the gas from D to E to F
Solution 1
Total work done by the gas from D to E to F = Area of ΔDEF
Area of ΔDEF =`1/2DExxEF`
Where,
DF = Change in pressure
= 600 N/m2 – 300 N/m2
= 300 N/m2
FE = Change in volume
= 5.0 m3 – 2.0 m3
= 3.0 m3
Area of ΔDEF =`1/2xx300xx3` = 450 J
Therefore, the total work done by the gas from D to E to F is 450 J.
Solution 2
As is clear figure
Change in pressure, `triangle P = EF = 5.0 - 2.0 = 3.0 atm = 3.0 xx 10^5 Nm^(-2)`
Change in volume. triangle V = DF = 600 - 300 = 300 cc
= `300 xx 10^(-6) m^3`
Work done by the gas from D to E to F = area of `triangle DEF`
`W =1/2 xx DF xx EF`
`= 1/2 xx (300 xx 10^(-6)) xx (3.0 xx 10^5) = 45 J`