Advertisements
Advertisements
Question
AB3 is an interhalogen T-shaped molecule. The number of lone pairs of electrons on A is ______. (Integer answer)
Options
9
6
2
0
MCQ
Fill in the Blanks
Solution
AB3 is an interhalogen T-shaped molecule. The number of lone pairs of electrons on A is 2.
Explanation:
AB3 represent sp3d hybridisation and it contains 3 bond pair and 2 lone pair.
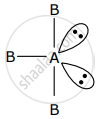
Number of lone pair = 2
shaalaa.com
Is there an error in this question or solution?
