Advertisements
Advertisements
Question
Ammonia burns in oxygen and the combustion, in the presence of a catalyst, may be represented by;
\[\ce{2NH3 + 2 1/2O2 -> 2NO + 3H2O}\] [H = 1, N = 14, O = 16]
What mass of steam is produced when 1.5 g of nitrogen monoxide is formed?
Solution
From equation, \[\ce{2NH3 + 2 1/2O2 -> 2NO + 3H2O}\]
When 60 g NO is formed, the mass of steam produced = 54 g
So, 1.5 g NO is formed, the mass of steam produced = `54 xx 1.5/60`
= 1.35 g
APPEARS IN
RELATED QUESTIONS
Give balanced chemical equations for the following conversions A, B, and C:
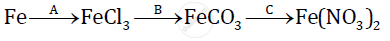
Calculate the relative molecular mass of Potassium chlorate.
(use K = 39, Cl = 35.5, O = 16, C = 12, H = 1, Na = 23, N = 14, S=32)
Calculate the percentage of water in ferrous sulphate crystals.
[Fe = 56, S = 32, O =16, H = 1].
Mention the term defined by the following sentence:
The mass of a given volume of gas compared to the mass of an equal volume of hydrogen.
A compound 'X' consists of 4.8% of C and 95.2% of Br by mass.
Name the type of chemical reaction by which X can be prepared from ethane.
Give one word or phrase for the following:
Formation of ions from molecules.
Define or explain the term:
Vapour density
Calculate the relative molecular mass of:
CuSO4. 5H2O
Which of the following contains maximum number of molecules?
Calculate the number of hydrogen atoms in 0.1 mole of H2SO4.
