Advertisements
Advertisements
Question
An organic compound [A], having a specific smell forms two compounds [B] and [C] by reacting with conc. sodium hydroxide. The molecular formula of compound [B] is C7H8O, which forms compound [A] again on oxidation. Compound [C] forms benzene on heating with soda lime.
Write the structures of compounds [A], [B] and [C]. Also, write the reactions involved.
Solution
The chemical component [A] must be an aromatic aldehyde, i.e., benzaldehyde with a distinct odour. When benzaldehyde is combined with NaOH. It undergoes Cannizzaro's reaction, yielding benzyl alcohol, [B] of the formula C7H8O and a sodium salt of benzoic acid [C]. Benzyl alcohol degrades into benzaldehyde [A] when oxidised. When heated with soda lime, the chemical [C] produces an aromatic hydrocarbon [D], which is benzene. The enters series of reaction is as given:
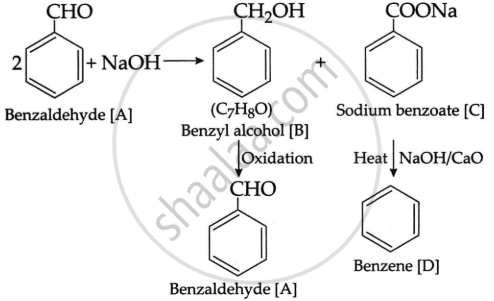
APPEARS IN
RELATED QUESTIONS
An organic compound A with molecular formula C2H7N on reaction with nitrous acid gives a compound B. Bon controlled oxidation gives compound C. C reduces Tollen's -reagent to give silver mirror and D. B reacts with D in the presence of concentrated sulphuric acid to give sweet smelling compound E. Identify A, B, C, D. and E. Give the reaction of C with ammonia
The compound which is optically active is:
Answer the following:
Name the two organic compounds which have the same molecular formula C2H6O. Will they react with PCl5? If they react, what are the products formed?
An organic compound [A] having molecular formula C2H7N on treatment with nitrous acid gives a compound [B] having molecular formula C2H6O. [B] on treatment with an organic compound [C] gives a carboxylic acid [D] and a sweet-smelling compound [E]. Oxidation of [B] with acidified potassium dichromate also gives [D].
(i) Identify [A], [B], [C], [D] and [E].
(ii) Write a balanced chemical equation of [D] with chlorine in the presence of red phosphorus and name the reaction.
An organic compound C2H4O gives a red precipitate when heated with Fehling solution. It also undergoes aldol condensation in the presence of dilute NaOH.
- Identify the organic compound and write its IUPAC name.
- Which compound will be formed when this organic compound reacts with hydroxylamine?
- What is observed when the compound, referred to in subpart (i), is heated with ammonical silver nitrate?
An organic compound [A] which has characteristic odour, reacts with conc. NaOH to give two compounds [B] and [C]. Compound [B] has molecular formula C7H8O which upon oxidation gives back compound [A]. Compound [C] is the sodium salt of the acid and upon treatment with sodalime yields an aromatic hydrocarbon [D]. Identify the compounds [A], [B], [C] and [D].
