ISC (Science)
Academic Year: 2023-2024
Date & Time: 26th February 2024, 2:00 pm
Duration: 3h
Advertisements
- Candidates are allowed an additional 15 minutes for only reading the paper.
- They must NOT start writing during this time.
- This paper is divided into four sections A, B, C and D.
- Answer all questions.
- Section A consists of one question having sub-parts of one mark each.
- Section B consists of ten questions of tuwo marks each.
- Section C consists of seven questions of three marks each and
- Section D consists of three quesfions of five marks each.
- Internal choices have been provided in one question each in Section B, Section C and Section D.
- All working, including rough work, should be done on the same sheet as, and adjacent to the rest of the answer.
- The intended marks for questions or parts of questions are given in brackets [].
- Balanced equations must be given wherever possible and diagrams where they are helpful.
- When solving numerical problems, all essential working must be shown.
- In working out problems, use the following data :
Gas constant R = 1.987 cal deg-1 mol-1 = 8.314 JK-1mol-1 = 0.0821 dm3 atm K-1 mol-1
1 l. 1 atm =1 dm3 atm = 101.3 J.
1 Faraday = 96500 coulombs.
Avogadro's number = 6.023 x 1023
For a particular reaction, the value of the rate constant is 0.05 sec−1. The reaction is of ______ order and will be ______ of the initial concentration.
lead poisoning
zero
phosgene
dependent
cancer
independent
diethyl ether
first
ethyl carbonate
ethene
Chapter: [0.031] Effect of concentration of reactants on the rate of a reaction
EDTA is used in the treatment of ______ while Cis platin is used in the treatment of ______.
lead poisoning
zero
phosgene
dependent
cancer
independent
diethyl ether
first
ethyl carbonate
ethene
Chapter: [0.030600000000000002] Catalyst
The addition of small quantity of ethanol to chloroform prevents the formation of ______ and converts it into the harmless compound ______.
lead poisoning
zero
phosgene
dependent
cancer
independent
diethyl ether
first
ethyl carbonate
ethene
Chapter: [0.115] Preparation, properties, and uses of the following
The dehydration of ethyl alcohol with conc. H2SO4 at 140 °C mainly yields ______ while at 170 °C the main product formed is ______.
lead poisoning
zero
phosgene
dependent
cancer
independent
diethyl ether
first
ethyl carbonate
ethene
Chapter: [0.121] Methods of preparation, manufacture, properties and use
Which one of the following statements is correct regarding the dry cell?
(P) Zinc container acts as an anode in dry cell.
(Q) Zinc container touches the paste of MnO2 and carbon.
(R) Dry cell can be charged easily.
(S) Graphite rod acts as a cathode in dry cell.
Only (P) and (R)
Only (Q) and (R)
Only (P) and (S)
Only (Q) and (S)
Chapter: [0.062] Galvanic cells, mechanism of current production in a galvanic cell;
The metal complex ion that is paramagnetic is ______.
(Atomic number of Fe = 26, Cu = 29, Co = 27 and Ni = 28)
[Fe(CN)4]2−
[Co(NH3)6]3+
[Ni(CN4)]2−
[Cu(NH3)4]2+
Chapter: [0.02] States of Matters: Structure and Properties Solid State
When KMnO4 is heated with acidified oxalic acid, gas bubbles are evolved. These gas bubbles are evolved due to the formation of ______.
SO2
CO2
SO3
O2
Chapter: [0.122] Preparation, properties and uses of ethane-1, 2 diol, propane-1, 2, 3 triol
The reaction of ethanamide with alcoholic sodium hydroxide and bromine gives ______.
Ethylamine
Methylamine
Propylamine
Aniline
Chapter: [0.113] Preparation, properties, uses of haloalkanes
An equimolar solution of non-volatile solutes A and B shows a depression in freezing point in the ratio of 2:1. If A remains in its normal state in the solution, the state of B in the solution will be ______.
Normal
Hydrolysed
Associated
Dissociated
Chapter: [0.013000000000000001] Relative molecular mass of non-volatile substances [0.013000000000000001] Relative molecular mass of non-volatile substances
Assertion: Specific conductivity of all electrolytes decreases on dilution.
Reason: On dilution, the number of ions per unit volume decreases.
Both Assertion and Reason are true and Reason is the correct explanation for Assertion.
Both Assertion and Reason are true but Reason is not the correct explanation for Assertion.
Assertion is true but Reason is false.
Assertion is false but Reason is true.
Chapter: [0.064] Electrolytic conductance
Assertion: Amimonolysis of alkyl halides involves the reaction between alkyl halides and alcoholic ammonia.
Reason: Ammonolysis of alkyl halides produces secondary amines only.
Both Assertion and Reason are true and Reason is the correct explanation for Assertion.
Both Assertion and Reason are true but Reason is not the correct explanation for Assertion.
Assertion is true but Reason is false.
Assertion is false but Reason is true.
Chapter: [0.15] Cyanides, Isocyanides, Nitro compounds, Amines and Diazonium Salt
Read the passage given below and answer the questions that follow.
| When two solutions are separated by a semi-permeable membrane, the solvent molecules move from a solution of lower molar concentration to a solution of higher molar concentration through osmosis. |
- Samar removed the outer hard shell of two different eggs while cooking at home. He then placed one egg in pure water and the other egg in a saturated solution of sucrose. What change is he likely to observe in the eggs after a few hours?
- Which solution, hypertonic or hypotonic, has a higher amount of solute in same quantity of solution?
- A 5% aqueous solution of glucose (molar mass = 180 g mol-1) is isotonic with 1.66 % aqueous solution of urea. Calculate the molar mass of urea.
Chapter: [0.0302] Collision Theory [0.0304] Effect of temperature on the rate constant of a reaction
Write a chemical test to distinguish between ethanol and phenol.
Chapter: [0.12300000000000001] Distinction between primary, secondary and tertiary alcohols
Give a chemical reaction to convert acetaldehyde into secondary propyl alcohol.
Chapter: [0.131] Carbonyl compounds
Give a reason for the following:
Zinc, cadmium and mercury are considered as d-block elements but not regarded as transition elements.
Chapter: [0.1] Chemistry of Transition and Inner-Transition Elements: d-Block: 3d, 4d and 5d series f-Block: 4f and 5f series
Give a reason for the following:
Transition metals possess a great tendency to form complex compounds.
Chapter: [0.1] Chemistry of Transition and Inner-Transition Elements: d-Block: 3d, 4d and 5d series f-Block: 4f and 5f series
Convert the following by giving a chemical equation:
Ethyl bromide to diethyl ether.
Chapter: [0.115] Preparation, properties, and uses of the following
Convert the following by giving a chemical equation:
Phenol to salicylaldehyde.
Chapter: [0.12300000000000001] Distinction between primary, secondary and tertiary alcohols
Account for the following:
Zirconium (Zr) and Hafnium (Hf) are difficult to separate.
Chapter: [0.1] Chemistry of Transition and Inner-Transition Elements: d-Block: 3d, 4d and 5d series f-Block: 4f and 5f series
Account for the following:
Salts of Cupric (Cu2+) ion are coloured whereas salts of the Cuprous (Cu+) ion are colourless.
Chapter: [0.053] Salt hydrolysis
Advertisements
How will you bring the following conversion?
Benzene to biphenyl
Chapter: [0.114] Chlorobenzene
How will you bring the following conversion?
Iodoform to acetylene
Chapter: [0.131] Carbonyl compounds
Calculate the maximum possible electrical work that can be obtained from a galvanic cell under standard conditions at 298K.
\[\ce{Zn|\underset{(aq)}{Zn}^2+ || \underset{(aq)}{Ag}^+ |Ag}\]
Given \[\ce{E°_{(Zn^2+/Zn)} = - 0.76V; E°_{(Ag+/Ag)} = + 0.80 V}\]
Chapter: [0.062] Galvanic cells, mechanism of current production in a galvanic cell;
Give a reason for the following:
Ethoxy ethane does not react with sodium, but ethanol does.
Chapter: [0.121] Methods of preparation, manufacture, properties and use
Give a reason for the following:
Ethoxy ethane with conc. HI at 373 K gives C2H5OH and CH3I but not CH3OH and C2H5I.
Chapter: [0.122] Preparation, properties and uses of ethane-1, 2 diol, propane-1, 2, 3 triol
An organic compound [A] having the molecular formula C4H10O forms a compound [B] with the molecular formula C4H8O on oxidation. Compound [B] gives a positive iodoform test. The reaction of compound [B] with CH3MgBr followed by hydrolysis, gives compound [C] with the molecular formula C5H12O. Identify the compounds [A], [B] and [C]. Write the reaction for the conversion of compound [A] to compound [B].
Chapter: [0.0311] Molecularity of the reaction
If 200 cm3 of an aqueous solution of a protein contains 1.26 g of protein, the osmotic pressure of the solution at 300K is found to be 2.57 × 10−3 atm.
Calculate the molar mass of protein.
(R = 0.0821 L atm K−1 mol−1)
Chapter: [0.013000000000000001] Relative molecular mass of non-volatile substances
Benzaldehyde is less reactive than propionaldehyde. Why?
Chapter: [0.131] Carbonyl compounds
In the preparation of ethanal by the oxidation of ethanol, ethanal should be removed immediately as it is formed. Why?
Chapter: [0.07] Coordination Compounds
Why is Mn2+ ion more stable than Fe2+ ion?
(Atomic numbers of Mn = 25 and Fe = 26)
Chapter: [0.1] Chemistry of Transition and Inner-Transition Elements: d-Block: 3d, 4d and 5d series f-Block: 4f and 5f series
Trivalent Lanthanoid ions such as La3+ (Z = 57) and Lu3+ (Z = 71) do not show any colour in their solution. Give a reason.
Chapter: [0.1] Chemistry of Transition and Inner-Transition Elements: d-Block: 3d, 4d and 5d series f-Block: 4f and 5f series
For the reaction \[\ce{A + B <=>}\] Product, the following data was obtained.
| Experiment number | Initial conception of [A] (mol L−1) | Initial concentration of [B] (mol L−1) | Initial Rate (mol L−1 minL−1) |
| 1 | 0.15 | 0.15 | 9.6 × 10−2 |
| 2 | 0.30 | 0.15 | 3.84 × 10−1 |
| 3 | 0.15 | 0.30 | 1.92 × 10−1 |
| 4 | 0.30 | 0.30 | 7.68 × 10−1 |
Calculate the following:
- The overall order of the reaction
- The rate law equation
- The value of rate constant
Chapter: [0.030299999999999997] Mechanism of the reaction
Illustrate the following reaction by giving one suitable example:
Coupling reaction
Chapter: [0.15] Cyanides, Isocyanides, Nitro compounds, Amines and Diazonium Salt
Illustrate the following reaction by giving one suitable example:
Acetylation of ethylamine
Chapter: [0.142] Acid derivatives
Aniline does not give Friedel-Crafts reaction. Give a reason.
Chapter: [0.15] Cyanides, Isocyanides, Nitro compounds, Amines and Diazonium Salt
Aradhana visits a physician as she is suffering from rickets and joint pain. Which fat-soluble vitamin should the physician prescribe to her?
Chapter: [0.17] Biomolecules – carbohydrates, proteins, enzymes, vitamins and nucleic acids
Somesh put a few drops of vinegar in the milk. What change do you think he observed in the milk after some time? What is this phenomenon known as?
Chapter: [0.052000000000000005] Ionic product of water, pH of solutions and pH indicators
Name the product of hydrolysis of sucrose. Is it a reducing sugar or a non-reducing sugar?
Chapter: [0.17] Biomolecules – carbohydrates, proteins, enzymes, vitamins and nucleic acids
An aqueous solution containing 12.50 g of barium chloride in 1000 g of water boils at 373.0834 K. Calculate the degree of dissociation of barium chloride.
Given Kb for H2O = 0.52 K kg mol−1; molecular mass of BaCl2 = 208.34 g mol−1.
Chapter: [0.013000000000000001] Relative molecular mass of non-volatile substances
Advertisements
An organic compound C2H4O gives a red precipitate when heated with Fehling solution. It also undergoes aldol condensation in the presence of dilute NaOH.
- Identify the organic compound and write its IUPAC name.
- Which compound will be formed when this organic compound reacts with hydroxylamine?
- What is observed when the compound, referred to in subpart (i), is heated with ammonical silver nitrate?
Chapter: [0.15] Cyanides, Isocyanides, Nitro compounds, Amines and Diazonium Salt
Identify the compound A,B and C:
\[\ce{C2H5OH->[PCl5]A->[KCN]B->[H3O+]C2H5COOH->[NH3][\Delta]C}\]
Chapter: [0.115] Preparation, properties, and uses of the following
Identify the compound [A], [B] and [C] in the following reaction.
\[\ce{C6H5OH ->[Zn/dust] [A] ->[CH3Cl][anhy.AlCl3] [B] ->[{[O]}][K2Cr2O7 + H2SO4] [C]}\]
Chapter: [0.141] Carboxylic acids
Give a chemical test to distinguish between the following pair of compound:
Ethanol and methanol
Chapter: [0.121] Methods of preparation, manufacture, properties and use
Give a chemical test to distinguish between the following pair of compound:
Ethanol and Ethanal
Chapter: [0.121] Methods of preparation, manufacture, properties and use
Give a chemical test to distinguish between the following pair of compound:
Propan-2-ol and 2-methyl propan-2-ol
Chapter: [0.121] Methods of preparation, manufacture, properties and use
The rate constant of a reaction at 500K and 700K are 0.02 sec−1 and 0.07 sec−1 respectively. Calculate the value of Ea. (activation energy)
Chapter: [0.0304] Effect of temperature on the rate constant of a reaction
A radioactive substance which emits alpha particle follows a first-order reaction. The half-life period of this radioactive substance is 30 hours. Calculate the fraction in percent (%) of the radioactive substance which remains after 90 hours.
Chapter: [0.015] Normality, molality
An organic compound [A], having a specific smell forms two compounds [B] and [C] by reacting with conc. sodium hydroxide. The molecular formula of compound [B] is C7H8O, which forms compound [A] again on oxidation. Compound [C] forms benzene on heating with soda lime.
Write the structures of compounds [A], [B] and [C]. Also, write the reactions involved.
Chapter: [0.15] Cyanides, Isocyanides, Nitro compounds, Amines and Diazonium Salt
Identify the compound [A] and [B] in the reaction given below:
\[\ce{C6H6 ->[CH3Cl][AlCl3(anhy.)] [A] ->[{[O]}][KrCr2O7 + H2SO4] [B]}\]
Chapter: [0.141] Carboxylic acids
Identify the compound [A] and [B] in the reaction given below:
\[\ce{CH3 - CHOH - CH3 ->[{[O]}][K2CrO7 + H2SO] [A] ->[NH2OH] [B]}\]
Chapter: [0.121] Methods of preparation, manufacture, properties and use
A coordination compound has the formula \[\ce{CoCl3.4NH3}\]. It precipitates silver ions as AgCl and its molar conductance corresponds to a total of two ions.
Based on this information, answer the following question:
- Deduce the structural formula of the complex compound.
- Write the IUPAC name of the complex compound.
- Draw the geometrical isomers of the complex compound.
Chapter: [0.1] Chemistry of Transition and Inner-Transition Elements: d-Block: 3d, 4d and 5d series f-Block: 4f and 5f series
Give a chemical test to show that \[\ce{[Co(NH3)5Cl]SO4}\] and \[\ce{[Co(NH3)5SO4]CI}\] are ionisation isomers.
Chapter: [0.07] Coordination Compounds
Study the diagram given below that represents Cu-Ag electrochemical cell and answer the questions that follow.
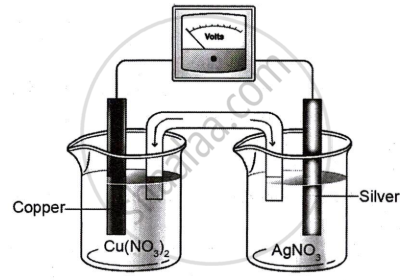
Given \[\ce{E^0_{(Cu^{2+}/Cu)}}\] = 0.337V; \[\ce{E^0_{(Ag^+/Ag)}}\] = 0.799V
- Write the cell reaction for the above cell.
- Calculate the standard emf of the cell.
- If the concentration of [Cu2+] is 0.1 M and Ecell is 0.422 V, at 25°C, calculate the concentration of [Ag+].
- Calculate ΔG for the cell.
Chapter: [0.062] Galvanic cells, mechanism of current production in a galvanic cell;
Calculate \[\ce{\wedge^0_m BaCl2 and Al2(SO4)}\] from the following data.
For \[\ce{\wedge^0_m Ba^2+ = 127.2 S cm^2 mol^{-1}, \wedge^0_m Al^3+ = 189 S cm^2 mol^{−1}}\]\[\ce{\wedge^0_m Cl= 76.3 S cm^2 mol^{-1}, \wedge^0_m SO^2-_4 = 160 S cm^2 mol^{-1}}\]
Chapter: [0.0311] Molecularity of the reaction
A 0.05 M NH4OH solution offers the resistance of 30.8 ohms to a conductivity cell at 298K. If the cell constant is 0.343 cm−1 and the molar conductance of NH4OH at infinite dilution is 471.4 S cm2 mol−1, calculate the following:
- Specific conductance
- Molar conductance
- Degree of dissociation
Chapter: [0.064] Electrolytic conductance
In the diagram of the electrolytic cell given below, A, B and C are connected in series having electrolytes of ZnSO4, AgNO3 and CuSO4, respectively.
A steady current of 1.5 A was passed until 1.45 g of Ag was deposited at the cathode of cell B.
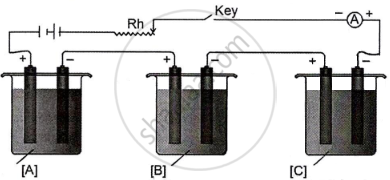
(Atomic mass of Ag = 108, Cu = 63.5, Zn = 65.3)
Answer the following questions:
- How long did the current flow?
- What weight of Cu and Zn was deposited at the cathode?
Chapter: [0.064] Electrolytic conductance
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
CISCE previous year question papers ISC Class 12 Chemistry (Theory) with solutions 2023 - 2024
Previous year Question paper for CISCE ISC Class 12 -2024 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Chemistry (Theory), you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of CISCE ISC Class 12.
How CISCE ISC Class 12 Question Paper solutions Help Students ?
• Question paper solutions for Chemistry (Theory) will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
