Advertisements
Advertisements
Question
In the diagram of the electrolytic cell given below, A, B and C are connected in series having electrolytes of ZnSO4, AgNO3 and CuSO4, respectively.
A steady current of 1.5 A was passed until 1.45 g of Ag was deposited at the cathode of cell B.
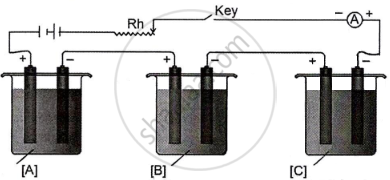
(Atomic mass of Ag = 108, Cu = 63.5, Zn = 65.3)
Answer the following questions:
- How long did the current flow?
- What weight of Cu and Zn was deposited at the cathode?
Solution
(1) Cell B
\[\ce{At Cathode - Ag+ + e- -> Ag(s)}\]
96500 C of current deposits 1 mole (108 g) of Ag.
1295.6 C of current will deposit 1.45 g of Ag.
Now, Q = it
1295.6 = 1.5 × t
t = 863.7 s
The current will flow for 863.7 seconds.
(2) Cell A
\[\ce{Zn^{+2} + 2e- -> Zn(s)}\]
2 moles of current deposits 65.3 g of Zn and 1295.6 C of current will deposit 0.438 g of Zn.
Cell C
\[\ce{Cu^{+2} + 2e- -> Cu(s)}\]
2 moles of current deposit 63.5 g of Cu, while 1295.6 C deposits 0.426 g of Cu.
APPEARS IN
RELATED QUESTIONS
Fill in the blanks by choosing the appropriate word/words from those given in the brackets:
The unit of conductance is ………. and that of specific conductance is ………..
(Henry’s, aldol condensation, absence, do not, ohm, Raoult’s, increases, common ion effect, easily, three, solubility product, ohm-1, two, four, ohm-1, cm2, Cannizzaro, ohm-1 cm-1, zero, decreases, presence)
A 0.05 M NH4OH solution offers the resistance of 50 ohms to a conductivity cell at 298 K. If the cell constant is 0.50 cm-1 and molar conductance of NH4OH at infinite dilution is 471.4 ohm-1 cm2 mol-1, calculate:
(i) Specific conductance
(ii) Molar conductance
(iii) Degree of dissociation
The molar conductance of a solution _______ with dilution, while its specific conductance _______ with dilution.
A 0.05 M NH4OH solution offers the resistance of 30.8 ohms to a conductivity cell at 298K. If the cell constant is 0.343 cm−1 and the molar conductance of NH4OH at infinite dilution is 471.4 S cm2 mol−1, calculate the following:
- Specific conductance
- Molar conductance
- Degree of dissociation
