Advertisements
Advertisements
Question
Aniline does not give Friedel-Crafts reaction. Give a reason.
Solution
Aniline does not undergo Friedel-Craft's reaction because the reaction takes place in the presence of AlCl3, but AlCl3 is a Lewis acid, whereas aniline is a strong basic. Thus, aniline interacts with AlCl3 to produce a salt.
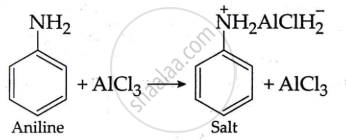
The positive charge on the N-atom prevents electrophilic substitution in the benzene ring. As a result, aniline does not undergo the Friedel-Craft reaction.
APPEARS IN
RELATED QUESTIONS
How is chlorobenzene prepared from aniline?
What is the action of acetic anhydride on ethylamine?
What is the action of the following reagents on aniline?
Bromine water
Write the structures of main products when aniline reacts with the following reagents :
Br2 water
Write the structures of main products when aniline reacts with the following reagents :
(CH3CO)2O/pyridine
Write the chemical equations involved when aniline is treated with the following reagents:
Br2 water
Write short notes on Acetylation
What is the action of acetic anhydride on diethylamine?
What is the action of the following reagents on aniline?
Acetic anhydride
What is the action of the following reagents on aniline?
Hot and conc. sulphuric acid
Illustrate the following reactions giving suitable example in each case
Acetylation of amines
How will you convert the following?
Aniline into N−phenylethanamide
In the nitration of benzene using a mixture of conc. \[\ce{H2SO4}\] and conc. \[\ce{HNO3}\], the species which initiates the reaction is ______.
What is the role of \[\ce{HNO3}\] in the nitrating mixture used for nitration of benzene?
Assertion: N, N-Diethylbenzene sulphonamide is insoluble in alkali.
Reason: Sulphonyl group attached to nitrogen atom is strong electron-withdrawing group.
Give reasons for the following observation:
Aniline does not react with methyl chloride in the presence of anhydrous AlCl3 catalyst.
Assertion (A): Bromination of benzoic acid, gives m-bromobenzoic acid.
Reason (R): Carboxyl group increases the electron density at the meta position.
