Advertisements
Advertisements
Question
Arrange the following compounds in increasing order of dipole moment.
\[\ce{CH3CH2CH3, CH3CH2NH2, CH3CH2OH}\]
Solution
\[\ce{CH3CH2CH3 < CH3CH2NH2 < CH3CH2OH}\]
Since O is more electronegative than N, therefore, dipole moment of ethyl alcohol is higher than that of ethylamine. Propane however, has the least dipole moment since it is almost a non-polar molecule.
APPEARS IN
RELATED QUESTIONS
Write the structures of main products when benzene diazonium chloride `(C_6H_5N_2^(+)Cl^-)`reacts with the following reagents:
CuCN/KCN
Write the structures of main products when benzene diazonium chloride `(C_6H_5N_2^(+)Cl^-)`reacts with the following reagents:
H2O
Write the structures of A, B and C in the following:
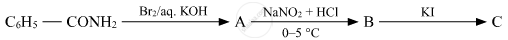
Give the structures of A, B and C in the following reaction:
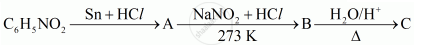
The reaction \[\ce{Ar\overset{+}{N2}Cl- ->[Cu/HCl] ArCl + N2 + CuCl}\] is anmed as ______.
Which of the following cannot be prepared by Sandmeyer’s reaction?
(i) Chlorobenzene
(ii) Bromobenzene
(iii) Iodobenzene
(iv) Fluorobenzene
Give reasons to support the answer:
3-Hydroxy pentan-2-one shows positive Tollen’s test.
Which of the following statements are correct:
(P) C6H5N=CH-C6H5 is a Schiff’s base.
(Q) A dye is obtained by the reaction of aniline and C6H5N=NCl.
(R) C6H5CH2NH2 on treatment with [NaNO2 + HCl] gives diazonium salt.
(S) p-Toluidine on treatment with [HNO2 + HCl] gives diazonium salt.
In a set of reactions, nitrobenzene gave a product D. Identify the product D.
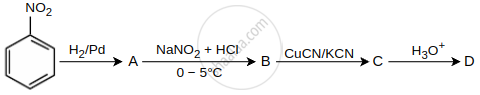
The conversion of benzene diazonium chloride to bromobenzene can be accomplished by ______.
