Advertisements
Advertisements
Question
Balance the following equation:
S + H2SO4 → SO2 + H2O
Solution
S + 2H2SO4 → 3SO2 + 2H2O
APPEARS IN
RELATED QUESTIONS
Translate the following statement into chemical equation and then balance the equation:
Barium chloride reacts with zinc sulphate to give zinc chloride and barium sulphate.
Substitute formulae for names and balance the following equation:
Calcium carbonate reacts with hydrochloric acid to produce calcium chloride, water and carbon dioxide gas.
When potassium nitrate is heated, it decomposes into potassium nitrite and oxygen. Write a balanced equation for this reaction and add the state symbols of the reactants and products.
One of the following is an exothermic reaction. This is:
(a) electrolysis of water
(b) conversion of limestone into quicklime
(c) process of respiration
(d) process of photosynthesis
Write the chemical equation for the following word equation and balance them.
Calcium oxide + Carbon dioxide → Calcium carbonate
In certain reaction an insoluble solid called precipitate is formed. State the colour and name of the precipitate formed in the following reaction involving addition of:
Copper [II] sulphate to sodium hydroxide.
Give word equation for the following chemical reaction and give the names of the product formed.
Zn + 2HC1→ ZnCl2 + H2
Name the following:
A catalyst which increases the rate of a chemical reaction.
Balance the following chemical equation.
- Na + O2 → Na2O3
- Ca + N2 → Ca3N2
- N2 + H2 →NH3
- CaCO3 +HCl → CaCl2 + CO2 +H2O
- Pb(NO3)2 → PbO + NO2 + O2
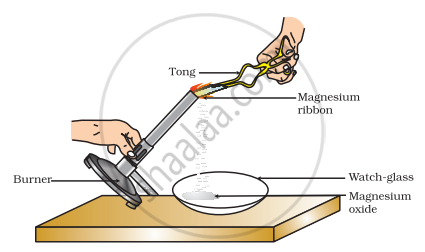
Which of the following is the correct observation of the reaction shown in the above set up?
