Advertisements
Advertisements
Question
Calculate the momentum of a photon of energy 6 x I 0-19 J.
Solution
The energy of a photon, E = p × e
6 ×10-19 = p × 3 × 108
∴ p = `(6xx10^(-19))/(3 xx 10^8) = 2 xx 10^(-27) Ns^(-1) `
APPEARS IN
RELATED QUESTIONS
The work functions for potassium and caesium are 2.25 eV and 2.14 eV respectively. Is the photoelectric effect possible for either of them if the incident wavelength is 5180 Å?
[Given : Planck’s constant = 6.63 x 10–34 J.s.;
Velocity of light = 3 x 108 m/s; 1 eV = 1.6 x 10–19 J]
In an experiment of the photoelectric effect, the graph of maximum kinetic energy EK of the emitted photoelectrons versus the frequency v of the incident light is a straight line AB shown in Figure 6 below:
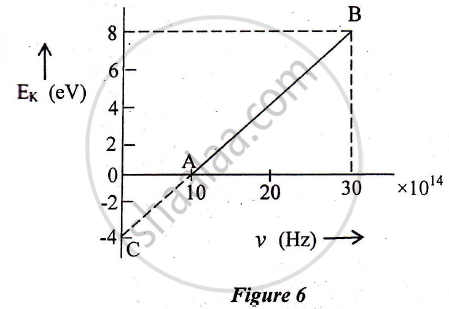
Find:
1) Threshold frequency of the metal
2) The work function of the metal.
3) Stopping potential for the photoelectrons emitted by the light of frequency `v = 30 xx 10^14 Hz`
Use Einstein's photoelectric equation to explain the observations from this graph ?
A beam of monochromatic radiation is incident on a photosensitive surface. Answer the following question giving reason :
Do the emitted photoelectrons have the same kinetic energy?
A beam of monochromatic radiation is incident on a photosensitive surface. Answer the following question giving reason :
On what factors does the number of emitted photoelectrons depend?
Draw a plot showing the variation of photoelectric current with collector plate potential for two different frequencies, v1 > v2, of incident radiation having the same intensity. In which case will the stopping potential be higher? Justify your answer.
With reference to the photoelectric effect, what is meant by threshold wavelength?
Two metals A and B have work functions 4 eV and 6 eV respectively. Which metal has a lower threshold wavelength for photoelectric effect?
Photoelectric effect is possible ______.
Light of wavelength 4000 Å is incident on two metals A and B. Which metal will emit photoelectrons, if their work functions are 3.8 e V and 1.6 e V respectively?
