Advertisements
Advertisements
Question
Calculate the number of unit cells in 0.3 g of a species having density of 8.5 g/cm3 and unit cell edge length 3.25 × 10-8 cm.
Solution
Given: Density (ρ) = 8.5 g/cm3, Edge length (a) = 3.25 × 10-8 cm, Mass of the species (x) = 0.3 g
To find: Number of unit cells in 0.3 g
Formula: Number of unit cells in x g of species = `x/(rho "a"^3)`
Calculation: Using formula,
Number of unit cells in 0.3 g of the species
`= (0.3 "g")/(8.5 "g cm"^-3 xx (3.25 xx 10^-8 "cm")^3)`
`= 1.03 xx 10^21`
Number of unit cells in 0.3 g of the given species is 1.03 × 1021.
RELATED QUESTIONS
Answer the following in brief.
Calculate the number of atoms in fcc unit cell.
Obtain the relationship between the density of a substance and the edge length of the unit cell.
Give the percentage of empty space in bcc lattice.
Derive the relationship between density of substance, its molar mass, and the unit cell edge length. Explain how you will calculate the number of particles, and a number of unit cells in x g of metal.
Silver crystallises in fcc structure, if edge length of unit cell is 316.5 pm. What is the radius of silver atom?
An element crystallizes in fcc type of unit cell. The volume of one unit cell is 24.99 × 10-24 cm3 and density of the element 7.2 g cm-3, Calculate the number of unit cells in 36 g of pure sample of element?
What is the percentage of unoccupied space in fcc unit cell?
The percentage of unoccupied volume in simple cubic cell is ______.
The number of atoms in 500 g of a fcc crystal of a metal with density d = 10 g/cm3 and cell edge 100 pm, is equal to ____________.
A metal crystallises in bcc unit cell with edge length 'a'. What will be the volume of one atom?
An element with density 2.8 g cm−3 forms fcc unit cell having edge length 4 × 10−8 cm. Calculate molar mass of the element.
Consider the following unit cell.
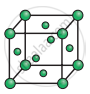
The number of particles (spheres) per unit cell is:
A metallic element has a cubic lattice with edge length of unit cell 2 Å. Calculate the number of unit cells in 200 g of the metal, if density of metal is 2.5 g cm-3?
Which of the following formulae is used to find edge length of bee unit cell?
The coordination number of atoms in body-centred cubic structure (bcc) is ______.
Gold crystallises into face-centred cubic cells. The edge length of a unit cell is 4.08 × 10–8 cm. Calculate the density of gold. [Molar mass of gold = 197 g mol–1]
A metal has an fcc lattice. The edge length of the unit cell is 404 pm. The density of the metal is 2.72 g cm−3. The molar mass of the metal is ______.
(NA Avogadro's constant = 6.02 × 1023 mol−1)
In face centred cubic unit cell, what is the volume occupied?
What is the density of potassium, if it has a bcc structure with edge length 4Å?
(Atomic mass of K = 39)
At room temperature, polonium Crystallises in a primitive cubic unit cell. If a = 3.36 Å. Calculate the theoretical density of polonium. [It's atomic weight is 209 g/mol.]
Silver crystallizes in the fcc structure. If the edge length of the unit cell is 400 pm, calculate the density of silver (Atomic mass of Ag = 108).
The correct sequence of the atomic layers in cubic close packing is ______.
The total number of different primitive unit cells is ______.
What would be the empirical formula of a compound having a unit cell containing A ion shared equally at the corner of the cube and B ion on the centre of faces of the cube?
An element crystallises in fee structure. If molar mass of element is 72.7 g mol -1, the mass of its one unit cell will be ______.
The number of particles present in Face Centred Cubic Unit cell is/are ______.
