Advertisements
Advertisements
Question
Choose the correct answer from the options given below: HCl gas can be prepared by direct combination of hydrogen and chlorine gas in presence of
Options
Direct sunlight
Dark atmosphere
Diffused sunlight
MnO2 catalyst
Solution
Diffused sunlight
APPEARS IN
RELATED QUESTIONS
When dilute HCl is added to a salt Q, a brisk effervescence is produced and the gas turns lime water milky.
When NH4OH solution is added to the above mixture (after adding dilute HCl), it produces a white precipitate which is soluble in excess NH4OH solution.
The aim of the Fountain experiment is to prove that ______.
Study the figure given below and answer the questions which follow:
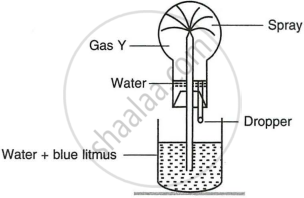
- Identify the gas Y.
- What property of gas Y does this experiment demonstrate?
- Name another gas which has the same property and can be demonstrated through this experiment.
Name the following:
Gas obtained by treating metals with hydrochloric acid.
Name the drying agent used to dry HCl gas.
How can you prove that hydrochloric acid contain :
Chlorine?
How will you show that hychloric acid contains both hydrogen and chlorine (other than by electrolysis) ?
Hydrogen chloride gas is very soluble in water. It is dissolved in water to prepare hydrochloric acid by using an anti-suction device, as shown in the diagram. Very briefly explain how this device prevents the suction of water into the flask, in which hydrogen chloride gas is produced.
- Name the experiment illustrated below.
- State the colour of the water that has entered the round-bottomed flask.
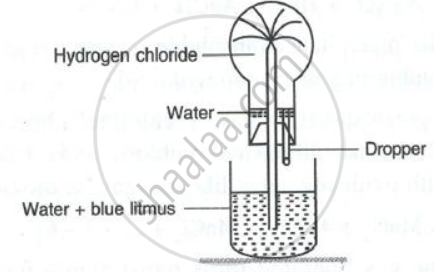
State the following:
The drying agent used in the laboratory preparation of HCl gas.
