Advertisements
Online Mock Tests
Chapters
2: Chemical Bonding
3: Study Of Acids, Bases and Salts
4: Analytical Chemistry
5: Mole Concept And Stoichiometry
6: Electrolysis
7: Metallurgy
▶ 8: Study of Compounds-I: Hydrogen Chloride
9.1: Ammonia
9.2: Nitric Acid
10: Study of Sulphur Compound: Sulphuric Acid
11.1: Organic Compounds
11.2: Alkanes
11.3: Unsaturated Hydrocarbons
11.4: Alkynes
11.5: Alcohols
11.6: Carboxylic Acid
12: Practical Work
![Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE chapter 8 - Study of Compounds-I: Hydrogen Chloride Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE chapter 8 - Study of Compounds-I: Hydrogen Chloride - Shaalaa.com](/images/chemistry-part-2-english-class-10-icse_6:083608507a004841af01e3c142179570.PNG)
Advertisements
Solutions for Chapter 8: Study of Compounds-I: Hydrogen Chloride
Below listed, you can find solutions for Chapter 8 of CISCE Frank for Chemistry - Part 2 [English] Class 10 ICSE.
Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE 8 Study of Compounds-I: Hydrogen Chloride Exercise 1 [Page 198]
Name the following :
Acid present in the stomach of mammals.
Name the following:
Drying agent used to dry hydrogen chloride.
Name the following:
Salt obtained by heating sodium chloride with concentrated sulphuric acid below 200 degree celcius.
Name the following:
Gas that yields white precipitates dense white fumes when mixed with hydrogen chloride.
Name the following:
Two solutions that yields white precipitates, when treated with hydrogen chloride or hydrochloric acid.
Name the following:
Gas obtained by treating metals with hydrochloric acid.
Name the following:
Gas obtained by treating ferrous sulphide with hydrochloric acid.
Name the following:
Five oxidizing agents that liberated chlorine from concentrated hydrochloric acid.
Name the following:
Acid used to extract glue from bones.
Name the following:
A chloride which is soluble in excess of ammonium hydroxide.
Name the following: A greenish yellow gas.
Name the following:
A chemical in which gold can be dissolved.
Name the following:
A metallic oxide which reacts with hydrochloric acid to give a coloured solution.
Name :
Two colourless gases which when mixed produce a white solid.
Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE 8 Study of Compounds-I: Hydrogen Chloride Exercise 2 [Page 199]
Calcium oxide and phosphorous pentoxide are very good drying agents but they are not used to dry HCI gas. Give reasons for each.
Draw a labelled diagram and explain the laboratory preparation of hydrogen chioride gas.
How will you prove that the gas prepared is HCI?
What is the function of HCI in preparation of aqua-regia?
Name the drying agent used to dry HCl gas.
How is the gas collected and why?
What are the important precautions?
How will you prepare hydrochloric acid in laboratory? OR
Describe briefly the method employed to dissolve hydrogen chloride gas in water as it is prepared. What is the purpose of funnel while preparing hydrochloric acid from HCI gas?
Explain, why (or give reasons for)
In the preparation chloride from sodium chloride, the gas can be obtained below 200°C or above. But the lower temperature is preferred.
Explain, why (or give reasons for)
Hydrogen chloride is not collected over water.
Explain, why (or give reasons for)
Hydrochloric acid cannot be concentrated above 22.2% by boiling.
Explain, why (or give reasons for)
HCI gas does not conduct electricity, but hydrochloric acid conducts electricity.
Explain, why (or give reasons for)
Only a very dilute solution of hydrogen chloride in water can be concentrated by boiling the solution while a very concentrated solution would become less concentrated when boiled.
Explain why when the stopper of a bottle full of hydrogen chloride gas is opened, there are fumes in the air.
Explain, why (or give reasons for)
A solution of HCl gas in water turns blue litmus red and conducts electricity, while HCI gas dissolves in toluene and it has no effect on litmus and does not conduct electricity.
Explain, why (or give reasons for)
An aqeous solution of chlorine is acidic is nature.
What would happen if
Calcium oxide is used to dry hydrogen chloride.
What would happen if :
Concentrated hydrochloric acid is kept open.
What would happen if
Hydrogen chloride prepared in laboratory, is passed through water, using a delivery tube.
Write three equations to show that hydrochloric acid acts as an acid.
Describe an experiment to prove the following:
HCI gas is heavier than air.
Describe an experiment to prove the following:
HCI is highly soluble in water.
Describe an experiment to prove the following:
HCI gas contains the element chlorine.
Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE 8 Study of Compounds-I: Hydrogen Chloride Exercise 3 [Page 200]
Name the gas evolved when dilute hydrochloric acid is added to: Zinc Metal
Name the gas evolved when dilute hydrochloric acid is added to: Calcium carbonate
Name the gas evolved when dilute hydrochloric acid is added to: Sodium sulphite
Name the gas evolved when dilute hydrochloric acid is added to: Lead (II) sulphide
Name the gas evolved when dilute hydrochloric acid is added to: Magnesium bicarbonate
Name the gas evolved when dilute hydrochloric acid is added to: Potassium bisulphite
Give a balanced equation when dilute hydrochloric acid is added to : Zinc Metal
Give a balanced equation when dilute hydrochloric acid is added to : Calcium carbonate
Give a balanced equation when dilute hydrochloric acid is added to : Sodium sulphite
Give a balanced equation when dilute hydrochloric acid is added to : Lead (II) sulphide
Give a balanced equation when dilute hydrochloric acid is added to : Magnesium bicarbonate
Give a balanced equation when dilute hydrochloric acid is added to : Potassium bisulphite
What is aqua-regia?
State the use of aqua-regia.
Give two tests for hydrochloric acid.
Write the uses of hydrochIoric acid.
Mention the reaction condition and give balanced equation to obtain: Cl2 gas from HCI gas.
Mention the reaction condition and give balanced equation to obtain: Cl2 gas from HCI acid.
Mention the reaction condition and give balanced equation to obtain : HCl gas from common salt
How can you prove that hydrochloric acid contain :
Hydrogen
How can you prove that hydrochloric acid contain :
Chlorine?
Fill in the blank:
Aqua-regia is a mixture of ___________ .
Fill in the blank:
A constant boiling mixture of water and hydrochloric acid is also called as _______________.
Fill in the blank:
___________ agent converts hydrochloric acid to chlorine.
Fill in the blank:
Hydrogen and chlorine reacts in presence of ___________ to form hydrogen chloride.
Fill in the blank:
On addition of silver nitrate to hydrochloric acid ___________ precipitate is formed which is soluble in ____________
Fill in the blank:
The white precipitate of lead chloride is soluble in _________
Choose the correct answer from the options given below: HCl gas can be prepared by direct combination of hydrogen and chlorine gas in presence of
Direct sunlight
Dark atmosphere
Diffused sunlight
MnO2 catalyst
Choose the correct answer from the options given below:
Dilute hydrochloric acid solution cannot be concentrated by boiling beyond
11%
33%
44%
22%
Choose the correct answer from the options given below:
Bleaching powder reacts with few drops of concentrated HCl to give
Chlorine
Calcium oxide
Oxygen
None of these
Choose the correct answer from the options given below:
Which of the following statement is not correct ?
HCl gas is collected by upward displacement of air
HCl acid gives white precipitate with AgNO3.
HCl gas is collected by downward displacement of air
HCl acid turns phenolphthalein solution colurless
Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE 8 Study of Compounds-I: Hydrogen Chloride Exercise 4 [Page 201]
Explain, why silver nitrate crystals are dissolved in distilled water and not in tap to prepare silver nitrate solution as a laboratory reagent.
Give one chemical test to distinguish between dilute hydrochloric acid and dilute sulphuric acid.
Name two gases which combine chemically to form a solid.
Name a chloride which is soluble in excess of ammonium hydroxide.
How will you show that hychloric acid contains both hydrogen and chlorine (other than by electrolysis) ?
Complete and balance the equations:
KMnO4 + HCI → _______+ _______ + _______ + ______ + 8H2O
Hydrogen chloride gas is very soluble in water. It is dissolved in water to prepare hydrochloric acid by using an anti-suction device, as shown in the diagram. Very briefly explain how this device prevents the suction of water into the flask, in which hydrogen chloride gas is produced.
Give reason for the following:
Dilute hydrochloric acid cannot be concentrated by distilling (boiling) the dilute acid.
Dilute hydrochloric acid is added in turn to a mixture of iron and sulphur and to the compound formed between iron and sulphur. Name the gas formed in each case.
Name the gas evolved when an oxide and concentrated hydrochloric acid are heated.
Explain, why the following statement is not correct:
Lead chloride can be prepared by adding dilute HCI to lead sulphate solution.
Outline the steps required to convert hydrogen chloride to anhydrous FeCl3. Write equations for the equations involved.
Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE 8 Study of Compounds-I: Hydrogen Chloride Exercise 5 [Page 202]
State what you see, when siIver nitrate solution is added to dilute HCl.
What must be added to sodium chloride to prepare hydrogen chloride?
Write the equation for the reaction which takes place in question(a).
What will you observe, when hydrogen chlorides mixed with ammonia?
Hydrogen chloride dissolves in water to form an acidic solution.
Name the experiment which demonstrates that hydrogen chloride is very soluble in water.
Hydrogen chloride dissolves in water to form an acidic solution.
Give three distinct tests (apart from using an indicator) you would carry out with this solution to illustrate the typical properties of an acid.
Write equation for the reaction between hydrochloric acid of the following: Bleaching powder
Write equation for the reaction between hydrochloric acid of the following: Lead nitrate solution
Write equation for the reaction between hydrochloric acid of the following: Manganese dioxide
Write equation for the reaction between hydrochloric acid of the following: Oxide of lead
What wilI you observed when concentrated HCI is added to lead (IV) oxide and warmed ?
Write balanced equation for the reaction between dilute hydrochloric acid and sodium sulphite.
Write the equation for :
The preparation of hydrogen chloride from sodium chloride and sulphuric acid. State whether the sulphuric acid should be concentrated or dilute.
Write the equation for:
The reaction between hydrogen chloride and ammonia.
Name one lead compound that can be used to oxidize hydrogen chioride to chIorine.
What happens when dilute hydrochloric acid is added to Iead nitrate solution ?
What happens when dilute hydrochloric acid is added to Iead nitrate solution ?
Write balanced equation for the following reaction:
Red lead (trilead tetroxide) is warmed with concentrated hydrochloric acid.
A solution of hydrogen chloride in water is prepared. The following substances are added to separate portions of the solution:
| S. No. | Substances added | Gas evolved | Odour |
| 1. | Calcium carbonate | _________ | _________ |
| 2. | Magnesium ribbon | _________ | _________ |
| 3. | Manganese (IV) oxide with heating | _________ | _________ |
| 4. | Sodium sulphide | _________ | _________ |
Complete the table by writing the gas evolved in each case and its odour.
Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE 8 Study of Compounds-I: Hydrogen Chloride Exercise 6 [Page 203]
State one reason why tap water is not used to prepare a solution of silver nitrate in the laboratory.
Write balanced equation for the following reaction:
Copper oxide and dilute hydrochloric acid.
Write balanced equcation for the following reaction:
Manganese (IV) oxide and concentrated hydrochloric acid.
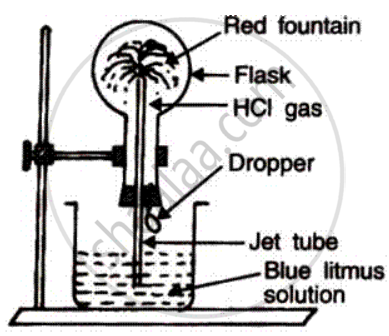
(a) Name the experiment illustrate above.
(b) Which property of hydrogen chloride is demonstrated by this experiment?
(c) State the colour of the water that has entered the round-bottomed flask.
Write a balanced equation for the reaction of zinc and dilute hydrochloric acid.
State what is observed when hydrochloric acid is added to silver nitrated solution.
of the two gases ammonia and hydrogen chloride, which is more dense. Name the method of collection of this gas.
Give one example of a reaction between the ammonia and hydrogen chloride gases which produces a solid
compound.
What is the property of concentrated sulphuric acid which allows it to be used in the preparation of hydrogen chloride and nitric acid?
What property of hydrogen chloride is demonstrated when it is collected by downward delivery (upward displacement) ?
Why is hydrogen chloride not collected over water?
Write a fully balanced of the following case :
Red lead is warmed with concentrated hydrochloric acid.
Write a fully balanced of the following case :
Magnesium metal is treated with dilute hydrochloric acid.
Correct the following statement:
Hydrochloric acid is prepared in the laboratory by passing hydrogen chloride directly through water.
Choose the correct answer from the option given below:
Aqua regia is a mixture of ______.
Dilute hydrochloric acid and concentrated nitric acid
Concentrated hydrochloric acid and dilute nitric acid
Concentrated hydrochloric acid [1 part] and concentrated nitric acid [3 parts]
Concentrated hydrochloric acid [3 parts] and concentrated nitric acid [1 part]
Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE 8 Study of Compounds-I: Hydrogen Chloride Exercise 7 [Page 204]

The diagram shows an apparatus for the laboratory preparation of hydrogen chloride.
(i) Identify A and B
(ii) Write the equation for the reaction
(iii) How would you check whether or not the gas jar is filled with hydrogen chloride?
(iv) What does the method of collection tell you about the density of hydrogen chloride.
By the addition of only one solution how would you distinguish between dilute hydrochloric acid and dilute nitric acid?
Choose the correct answer from the options given below:
Hydrogen chloride gas, being highly soluble in water, is dried by ______.
Anhydrous calcium chloride
Phosphorous pentaoxide
Quick time
Concentrated sulphuric acid
In the laboratory preparation of hydrochloric acid, \[\ce{HCl}\] gas is dissolved in water.
- Draw a diagram to show the arrangement used for the absorption of \[\ce{HCl}\] gas in water.
- State why such an arrangement is necessary? Give two reasons for the same.
- Write the chemical equation for the laboratory preparation of \[\ce{HCl}\] gas when the reactants are:
- Below 200°C
- Above 200°C
State one appropriate observation of the following :
Copper sulphate is treated with dilute hydrochloric acid
State one appropriate observation of the following:
A few drops of dilute hydrochloric acid are added to silver nitrate solution, followed by the addition of ammonium hydroxide solution.
State one appropriate observation of the following:
Which gas is evolved when potassium sulphite with dilute hydrochloric acid.
State one appropriate observation of the following:
Concentrated HCl is made to react with mangesediaoxide
State one appropriate observation of the following:
Action of dilute HCl or sodium sulphite.
Study the figure given below and answer that questions that follow: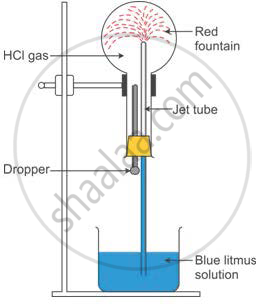
(i) Identify the gas Y.
(ii) What property of gas Y does this experiment demonstrate?
(iii) Name another gas which has the same property and can be demonstrated through this experiment.
Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE 8 Study of Compounds-I: Hydrogen Chloride Exercise 8 [Page 205]
The following question is pertaining to the laboratory preparation of hydrogen chloride gas.
Write the equation for its preparation mentioning the condition required.
The following question is pertaining to the laboratory pertaining hydrogen chloride gas.
Name the drying agent used and justify your choice.
Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE 8 Study of Compounds-I: Hydrogen Chloride Exercise 9 [Page 205]
The following question is pertaining to the laboratory pertaining hydrogen chloride gas.
State a safety precaution you would take during the preparation of hydrochloric acid.
The aim of the fountain experiment is to prove that
HCl turns blue litmus red
HCl is denser than air
HCl is highly soluble in water
HCl fumes in moist air
State your observation when dilute hydrochloric acid is added to lead nitrate solution and the mixture is heated.
State your observation when dilute hydrochloric acid is added to sodium thisulphate.
State your observation when :
Dilute hydrochloric acid is added to copper carbonate.
State the observation for action of dilute hydrochloiric acid or iron (II) sulphate.
How will you distinguished between dilute HCl and dilute H2SO4 using lead nitrate solution?
Solutions for 8: Study of Compounds-I: Hydrogen Chloride
![Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE chapter 8 - Study of Compounds-I: Hydrogen Chloride Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE chapter 8 - Study of Compounds-I: Hydrogen Chloride - Shaalaa.com](/images/chemistry-part-2-english-class-10-icse_6:083608507a004841af01e3c142179570.PNG)
Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE chapter 8 - Study of Compounds-I: Hydrogen Chloride
Shaalaa.com has the CISCE Mathematics Chemistry - Part 2 [English] Class 10 ICSE CISCE solutions in a manner that help students grasp basic concepts better and faster. The detailed, step-by-step solutions will help you understand the concepts better and clarify any confusion. Frank solutions for Mathematics Chemistry - Part 2 [English] Class 10 ICSE CISCE 8 (Study of Compounds-I: Hydrogen Chloride) include all questions with answers and detailed explanations. This will clear students' doubts about questions and improve their application skills while preparing for board exams.
Further, we at Shaalaa.com provide such solutions so students can prepare for written exams. Frank textbook solutions can be a core help for self-study and provide excellent self-help guidance for students.
Concepts covered in Chemistry - Part 2 [English] Class 10 ICSE chapter 8 Study of Compounds-I: Hydrogen Chloride are Hydrogen Chloride, Hydrochloric Acid, Uses of Hydrochloric Acid, General Preparation of Hydrogen Chloride Gas, Laboratory Preparation of Hydrogen Chloride Gas, Physical Properties of Hydrogen Chloride Gas, Chemical Properties of Hydrogen Chloride Gas, Laboratory Method of Preparation of Hydrochloric Acid, Properties of Hydrochloric Acid, Tests for Hydrogen Chloride and Hydrochloric Acid.
Using Frank Chemistry - Part 2 [English] Class 10 ICSE solutions Study of Compounds-I: Hydrogen Chloride exercise by students is an easy way to prepare for the exams, as they involve solutions arranged chapter-wise and also page-wise. The questions involved in Frank Solutions are essential questions that can be asked in the final exam. Maximum CISCE Chemistry - Part 2 [English] Class 10 ICSE students prefer Frank Textbook Solutions to score more in exams.
Get the free view of Chapter 8, Study of Compounds-I: Hydrogen Chloride Chemistry - Part 2 [English] Class 10 ICSE additional questions for Mathematics Chemistry - Part 2 [English] Class 10 ICSE CISCE, and you can use Shaalaa.com to keep it handy for your exam preparation.
