Advertisements
Online Mock Tests
Chapters
2: Chemical Bonding
3: Study Of Acids, Bases and Salts
4: Analytical Chemistry
5: Mole Concept And Stoichiometry
6: Electrolysis
7: Metallurgy
8: Study of Compounds-I: Hydrogen Chloride
9.1: Ammonia
9.2: Nitric Acid
▶ 10: Study of Sulphur Compound: Sulphuric Acid
11.1: Organic Compounds
11.2: Alkanes
11.3: Unsaturated Hydrocarbons
11.4: Alkynes
11.5: Alcohols
11.6: Carboxylic Acid
12: Practical Work
![Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE chapter 10 - Study of Sulphur Compound: Sulphuric Acid Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE chapter 10 - Study of Sulphur Compound: Sulphuric Acid - Shaalaa.com](/images/chemistry-part-2-english-class-10-icse_6:083608507a004841af01e3c142179570.PNG)
Advertisements
Solutions for Chapter 10: Study of Sulphur Compound: Sulphuric Acid
Below listed, you can find solutions for Chapter 10 of CISCE Frank for Chemistry - Part 2 [English] Class 10 ICSE.
Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE 10 Study of Sulphur Compound: Sulphuric Acid Exercise 1 [Page 249]
How does sulphuric acid occur in nature?
Describe the theory of manufacture of sulphuric acid by contact process, with all the necessary conditions (No technical details are required but equations should be given for each stage of process).
Define the following term:
Constant boiling mixture
Define the following term : Hygroscopic substance
Define the following term : Oleum
Define the following term : Dehydrating agent
Name the following:
Products obtained by dissolving sulphur dioxide and chlorine in water.
Name the following:
The catalyst used in the constant process.
Name the following:
Solution obtained by dissolving sulphur trioxide in 98% sulphuric acid.
Name the following:
Products obtained by treating zinc with dilute sulphuric acid.
Name the following:
Products obtained by treating ferrous sulphide with dilute sulphuric acid.
Name the following :
The precipitate obtained by treating aqueous lead nitrate with dilute sulphuric acid.
Name the following:
The precipitate obtained by treating aqueous barium chloride with dilute sulphuric acid.
Name the following:
The precipitate obtained by treating carbon with hot concentrated sulphuric acid.
Name the Following:
The property used to prepare HCl and HNO3 from H2SO4.
Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE 10 Study of Sulphur Compound: Sulphuric Acid Exercise 2 [Page 250]
What would you observe in the following case?
Sulphur trioxide is dissolved in water on a large scale.
What would you observe in the following case?
Concentrated sulphuric acid is added to equal volumes of cold water.
What would you observe in the following case?
100ml of 98% sulphuric acid is kept open.
What would you observe in the following case?
Hot concentrated sulphuric acid is added to sodium chloride crystals.
What would you observe in the following case?
Dilute sulphuric acid is added to ferrous sulphate solution.
Give reason for the following:
In the contact process, sulphur dioxide is dissolved in 98% sulphuric acid and not in water.
Give reason for the following:
When solution of sulphur dioxide is exposed to air, it gets converted to sulphuric acid.
Give reason for the following:
When diluting concentrated sulphuric acid, the acid should be added to water and not water to the acid.
Give reason for the following:
When concentrated sulphuric acid is exposed to air, its volume increases and it becomes slightly dilute.
Give reason for the following:
Sulphuric acid can form two kinds of salts with sodium chloride.
Give reason for the following:
When barium chloride is added to dilute sulphuric acid, a white precipitate is formed.
Give reason for the following:
When carbon is heated with concentrated sulphuric acid, carbon dioxide is formed.
Give reason for the following:
Ammonia gas cannot be dried by passing through concentrated sulphuric acid.
Give reason for the following:
When concentrated sulphuric acid is added to sugar/glucose, a black mass is left behind.
Give reason for the following:
Concentrated sulphuric acid should not be added to oxalic acid or formic acid in the open laboratory.
Give reason for the following:
When concentrated sulphuric acid is added to blue crystalline copper sulphate, it turns powdery white.
Give reason for the following:
Concentrated sulphuric acid must be stored in airtight bottles.
Give reason for the following:
Cotton clothes get burnt with concentrated sulphuric acid.
Give reason for the following:
H2SO4 cannot be prepared by heating sodium sulphate with conc. HCl or HNO3
How are the following conversions brought about? Give equation and condition:
Sulphur trioxide to sulphur acid.
How are the following conversion brought about? Give equation and condition:
Sulphur trioxide to oleum.
How are the following conversion brought about? Give equation and condition:
Dilute sulphuric acid to hydrogen.
How are the following conversion brought about? Give equation and condition:
Aqueous barium chloride to barium sulphate.
How are the following conversion brought about? Give equation and condition:
Aqueous lead nitrate to lead sulphate.
How are the following conversion brought about? Give equation and condition:
Sodium chloride to hydrogen chloride.
How are the following conversion brought about? Give equation and condition:
Sucrose to sugar charcoal.
How are the following conversion brought about? Give equation and condition:
Oxalic acid to carbon monoxide.
Describe the reaction that show
Concentrated sulphuric acid is a non-volatile acid.
Describe the reaction that show
Concentrated sulphuric acid is a dehydrating agent.
Describe the reaction that show
Concentrated sulphuric acid behaves as oxidizing agent.
Describe the reaction that show
Dilute sulphuric acid behaves as dibasic acid.
Why is sulphuric acid known as king of chemicals and oil vitriol ?
The following statement is correct only under certain conditions. Rewrite the statement including the appropriate conditions.
Oxalic acid reacts with sulphuric acid to produce carbon monoxide and carbon dioxide.
Give examples of the use of sulphuric acid as
An electrolyte in everyday use
Give examples of the use of sulphuric acid as
Non-volatile acid
Give examples of the use of sulphuric acid as
An oxidizing agent
How will you distinguish between dilute H2SO4 and conc. H2SO4 ?
How will you distinguish between conc. HCl and conc. H2SO4?
Give main difference between drying agent and dehydrating agent.
Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE 10 Study of Sulphur Compound: Sulphuric Acid Exercise 3 [Page 251]
Choose the current answer from the options given below :
In the preparation of H2SO4 by contact process V2O3 is used as a catalyst in the reaction. It is
S + O2→ SO2
SO2 + H2SO4→ H2S2O7
SO3 + H2O → H2SO4
2SO2 + O2→ 2SO3
Choose the current answer from the options given below :
When conc. H2SO4 comes in contact with sugar, it becomes black due to
Hydrolysis
Decolourisation
Dehydration
Hydration
Choose the current answer from the options given below :
Which of the following gas dissolves in H2SO4 to give alum?
SO2
H2S
S2O
SO3
Choose the current answer from the options given below :
In the contact process, the impurities of arsenic are removed by
Fe2O3
Fe(OH)3
AlCOH3
Cr(OH)3
Choose the current answer from the options given below :
The catalyst used for the oxidation of SO2 to SO3 in contact process is
Finally divided iron
Molybdenum
Vanadium pentoxide
Nitric oxide
Fill in the blank with appropriate word/words :
The catalyst used in contact process for the manufacture of H2SO4 is ___________ or _________.
Fill in the blank with appropriate word/words :
For the reaction SO2 + O2⇌ SO3 + Heat, the favourable conditions are _________ and __________.
Fill in the blank with appropriate word/words :
Oil of Vitriol is ___________
Give reason for the following:
Concentrated sulphuric acid is kept in airtight bottles.
Name the anion [negative ion] present in the following compound:
Compound D when warmed with dilute H2SO4 gives a gas which turns acidified potassium dichromate solution green.
What is the purpose of the contact process?
Name the two gases that are combined during the contact process.
Name the catalyst used in the process.
Write the equation for the reaction between zinc and the final product of the contact process.
Name two other acids other than sulphuric acid, which can be prepared by using sulphuric acid.
In using sulphuric acid to prepare other acids, as mentioned above, which property of sulphuric acid is used ?
Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE 10 Study of Sulphur Compound: Sulphuric Acid Exercise 4 [Page 252]
Sulphuric acid can be used to prepare a number of gases in the laboratory. Write balanced equation for the reaction from which the folllowing gas are obtained, using dilute sulphuric acid as one of the reactant : Hydrogen
Sulphuric acid can be used to prepare a number of gases in the laboratory. Write balanced equation for the reaction from which the folllowing gas are obtained, using dilute sulphuric acid as one of the reactant : Carbon dioxide
Sulphuric acid can be used to prepare a number of gases in the laboratory. Write balanced equation for the reaction from which the folllowing gas are obtained, using dilute sulphuric acid as one of the reactant : Sulphur dioxide
What would you see, when a crystal of CuSO4.5H2O is placed in concentrated sulphuric acid? Give reason to explain your observation.
Write balanced equations for the three chemical reactions that take place during the conversion of sulphur dioxide to sulphuric acid in the contact process. Name the catalyst used in the contact process.
What do you observe when barium chloride solution is added to dilute sulphuric acid ?
Write balanced equation for the reaction between iron and dilute sulphuric acid.
Name the oxide of sulphur which reacts water to give sulphuric acid.
In the contact process, the direct reaction between oxide of sulphur and water is avoided. In this process what does the oxide of sulphur react with instead of water, and what is the name of the product?
Write balanced equation for the reaction of dilute sulphuric acid with the following:
Copper carbonate
Write balanced equation for the reaction of dilute sulphuric acid with the following:
Lead nitrate solution
Write balanced equation for the reaction of dilute sulphuric acid with the following:
Zinc hydroxide
In this question, you required to supply the word (or words) that will make the sentence correct. Rewrite the copper statement.
Copper sulphate crystals are dehydrate by sulphuric acid.
Write balanced chemical equation for the reaction between zinc and dilute sulphuric acid.
(i) What is the purpose of the contact process ?
(ii) Name the catalyst used in the contact process.
(iii) Write the balanced equation for the reaction in the contact process, which takes place in the presence of catalyst.
What do you see when concentrated sulphuric acid is added to copper sulphate-5-water ( You are not required to say what is happing, nor is it necessary to name the products.)
Copy and complete the following table.
Column 3 has the names of gases to be prepared using the substance you enter in column 1, along with dilute or concentrated sulphuric acid, as indicated in column 2.
| Column 1 | Column 2 | Column 3 |
| Substance reacted with acid | Dilute or concentrated sulphuric acid | Gas |
| Hydrogen | ||
| Carbon dioxide | ||
| Only chlorine |
Write the equation for the laboratory preparation of :
(i) Sodium sulphate using dilute sulphuric acid.
(ii) Lead sulphate using dilute sulphuric acid.
Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE 10 Study of Sulphur Compound: Sulphuric Acid Exercise 5 [Page 253]
Which concentrated acid oxidizes sulphur directly to sulphuric acid ? Write the equation for the same. What is the name of the process by which sulphuric acid is manufactured ? Name the catalyst used in the process.
Complete the following sentence, choosing the correct word from the given options.
"Concentrated sulphuric acid is used in the laboratory preparation of nitric acid and hydrochloric acid because it is ____________ in comparison to these two acids."
less volatile
stronger
Write the equation for the laboratory preparation of the following salts, using sulphuric acid.
Iron (II) sulphate from iron.
Write the equation for the laboratory preparation of the following salts, using sulphuric acid.
Copper sulphate from copper.
Write the equation for the laboratory preparation of the following salts, using sulphuric acid.
Lead sulphate from lead nitrate.
Write the equation for the laboratory preparation of the following salts, using sulphuric acid.
Sodium Sulphate from sodium carbonate.
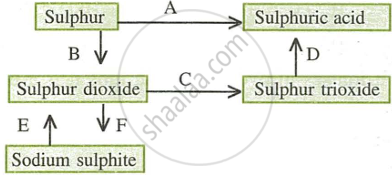
- Name the catalyst which helps in the conversion of sulphur dioxide to sulphur trioxide in step C.
- In the contact process for the manufacture of sulphuric acid, sulphur trioxide is not converted to sulphuric acid by reacting it with water. Instead a two-step procedure is used. Write the equations for the two steps involved in D.
- What type of substance will liberate sulphur dioxide from sodium sulphite in step E?
- Write the equation for the reaction by which sulphur dioxide is converted to sodium sulphite in step F.
Write balanced equation for potassium hydrogen carbonate and dilute sulphuric acid.
A, B, C and D summarize the properties of sulphuric acid depending on whether it is dilute or concentrated. Choose the property (A, B, C or D), depending on which is relevant to each of the preparations (i) to (ii).
A. Dilute acid (typical acid properties)
B. Non-volatile acid
C. Oxidizing agent
D. Dehydrating agent
(i) Preparation of hydrogen chloride.
(ii) Preparation of ethane from ethanol
(iii) Preparation of copper sulphate from copper oxide.
Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE 10 Study of Sulphur Compound: Sulphuric Acid Exercise 6 [Page 254]
Name the process used for the large scale manufacture of sulphuric acid.
Which property of sulphuric acid accounts for its use as a dehydrating agent ?
Concentrated sulphuric acid is both an oxidizing agent and a non-volatile acid. Write one equation each to illustrate the properties of sulphuric acid mentioned above.
Write the balanced chemical equation for the following conversion:
Lead sulphate from lead nitrate solution and dilute sulphuric acid.
Write the balanced chemical equation for the following conversion:
Copper sulphate from copper and concentrated sulphuric acid.
Write the balanced chemical equation for the following conversion:
Ammonium sulphate from ammonia and dilute sulphuric acid.
Some properties of sulphuric acid are listed below. Choose the property A, B, C or D which is responsible for the reaction (a) to (e). Some properties may be repeated.
A. Acid
B. Dehydrating agent
C. Non-volatile acid
D. Oxidizing agent
(a) \[\ce{C12H22O11 + nH2SO4 -> 12C + 11H2O + nH2SO4}\]
(b) \[\ce{S + 2H2SO4 -> 3SO2 + 2H2O}\]
(c) \[\ce{NaCl + 2H2SO4 -> NaHSO4 + HCl}\]
(d) \[\ce{CuO + 2H2SO4 -> CuSO4 + H2O}\]
(e) \[\ce{Na2CO3 + H2SO4 -> Na2SO4 + H2O + CO2}\]
HCl, HNO3 and H2SO4 are formulae of three compounds. Which of these compounds has the highest boiling point and which has the lowest?
Dilute hydrochloric acid and dilute sulphuric acid are both colourless solutions. How will the addition of barium chloride solution to each help to distinguish between the two?
Dilute sulphuric acid will produce a white precipitate when added to a solution of ______.
Copper nitrate
Zinc nitrate
Lead nitrate
Sodium nitrate
Making use only of substances chosen from those given below:
Dilute sulphuric acid
Sodium carbonate
Zinc
Sodium sulphite
Lead
Salcium carbonate
Give the equation for the reaction by which you could obtain:
(i) Hydrogen
(ii) Sulphur dioxide
(iii) Carbon dioxide
(iv) Zinc Carbonate (two steps required).
Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE 10 Study of Sulphur Compound: Sulphuric Acid Exercise 7 [Page 255]
Name the gas evolved in following case:
The gas produced by the action of concentrated sulphuric acid on sodium chloride.
Write the equation of the following reaction:
Sulphur is heated with concentrated sulphuric acid
Write the equation of the following reaction :
Concentrated sulphuric acid is poured over sugar
State your observation for the following case :
Moist blue litmus is introduced into a gas jar of sulphur dioxide.
State your observation for the following case :
Dry red rose petals are placed in the jar of sulphur dioxide.
State your observation for the following case :
Paper soaked in potassium permanganate solution is introduced into a gas jar of sulphur dioxide.
What would you observe in the following case:
Sugar crystals are added to a hard glass test tube containing concentrated sulphuric acid.
With the help of equations, give an outline for the manufacture of sulphuric acid by the contact process.
What property of sulphuric acid is shown by the reaction of concentrated sulphuric acid when heated with :
(i) Potassium nitrate
(ii) carbon
In the given equation identify the role played by concentrated sulphuric acid S + 2H2SO4→ 3SO2 + 2H2O
Non-volatile acid
Oxidising agent
Dehydrating agent
None of the above
State one appropriate observation of the following:
Concentrated sulphuric acid is added dropwise to a crystal of hydrated copper sulphate.
State one appropriate observation of the following:
Dehydration of Concentrated Sulphuric acid with sugar crystals
Give one equation to show the following properties of sulphuric acid : Dehydrating property
Give one equation to show the following property of sulphuric acid:
Acidic nature
Give one equation to show the following properties of sulphuric acid:
As a non-volatile acid
Give balanced chemical equation for the action of sulphuric acid of the following:
Potassium hydrogen carbonate
Give a balanced chemical equation for the action of sulphuric acid of the following:
Sulphur
In the contact process for the manufacture of sulphuric acid give the equations for the conversion of sulphur trioxide to sulphuric acid.
Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE 10 Study of Sulphur Compound: Sulphuric Acid Exercise 8 [Page 256]
A, B, C and D summarise the properties of sulphuric acid depending on whether it is dilute or concentrated.
A = Typical acid property
B = Non-Volatile acid
C = Oxidising agent
D = Dehydrating agent
Choose the property (A, B, C or D) depending on which is relevant to each of the following :
(i) Preparation of hydrogen chloride gas
(ii) Preparation of copper sulphate from copper oxide
(iii) Action of cone, sulphuric acid on sulphur
Write balanced chemical equation to show :
The oxidizing action of conc.sulphuric acid on carbon
Write balanced chemical equation to show :
The behavior of H2SO4 as an acid when it reacts with magnesium.
Write balanced chemical equation to show :
The dehydrating property of conc.sulphuric acid
Solutions for 10: Study of Sulphur Compound: Sulphuric Acid
![Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE chapter 10 - Study of Sulphur Compound: Sulphuric Acid Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE chapter 10 - Study of Sulphur Compound: Sulphuric Acid - Shaalaa.com](/images/chemistry-part-2-english-class-10-icse_6:083608507a004841af01e3c142179570.PNG)
Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE chapter 10 - Study of Sulphur Compound: Sulphuric Acid
Shaalaa.com has the CISCE Mathematics Chemistry - Part 2 [English] Class 10 ICSE CISCE solutions in a manner that help students grasp basic concepts better and faster. The detailed, step-by-step solutions will help you understand the concepts better and clarify any confusion. Frank solutions for Mathematics Chemistry - Part 2 [English] Class 10 ICSE CISCE 10 (Study of Sulphur Compound: Sulphuric Acid) include all questions with answers and detailed explanations. This will clear students' doubts about questions and improve their application skills while preparing for board exams.
Further, we at Shaalaa.com provide such solutions so students can prepare for written exams. Frank textbook solutions can be a core help for self-study and provide excellent self-help guidance for students.
Concepts covered in Chemistry - Part 2 [English] Class 10 ICSE chapter 10 Study of Sulphur Compound: Sulphuric Acid are Sulphuric Acid, Manufacture of Sulphuric Acid (Constant Process), Chemical Properties of Sulphuric Acid, Preparation of Sulphuric Acid, Physical Properties of Sulphuric Acid, Uses of Sulphuric Acid, Tests for Sulphuric Acid and Sulphates.
Using Frank Chemistry - Part 2 [English] Class 10 ICSE solutions Study of Sulphur Compound: Sulphuric Acid exercise by students is an easy way to prepare for the exams, as they involve solutions arranged chapter-wise and also page-wise. The questions involved in Frank Solutions are essential questions that can be asked in the final exam. Maximum CISCE Chemistry - Part 2 [English] Class 10 ICSE students prefer Frank Textbook Solutions to score more in exams.
Get the free view of Chapter 10, Study of Sulphur Compound: Sulphuric Acid Chemistry - Part 2 [English] Class 10 ICSE additional questions for Mathematics Chemistry - Part 2 [English] Class 10 ICSE CISCE, and you can use Shaalaa.com to keep it handy for your exam preparation.
