Advertisements
Online Mock Tests
Chapters
2: Chemical Bonding
3: Study Of Acids, Bases and Salts
4: Analytical Chemistry
5: Mole Concept And Stoichiometry
6: Electrolysis
7: Metallurgy
8: Study of Compounds-I: Hydrogen Chloride
▶ 9.1: Ammonia
9.2: Nitric Acid
10: Study of Sulphur Compound: Sulphuric Acid
11.1: Organic Compounds
11.2: Alkanes
11.3: Unsaturated Hydrocarbons
11.4: Alkynes
11.5: Alcohols
11.6: Carboxylic Acid
12: Practical Work
![Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE chapter 9.1 - Ammonia Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE chapter 9.1 - Ammonia - Shaalaa.com](/images/chemistry-part-2-english-class-10-icse_6:083608507a004841af01e3c142179570.PNG)
Advertisements
Solutions for Chapter 9.1: Ammonia
Below listed, you can find solutions for Chapter 9.1 of CISCE Frank for Chemistry - Part 2 [English] Class 10 ICSE.
Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE 9.1 Ammonia Exercise 1 [Page 219]
Give the formula of (i) Liquid ammonia (ii) Liquor ammonia
What is liquor ammonia fortis?
How is ammonia gas prepared in laboratory starting from NH4Cl? State the conditions and balanced equation for the preparation.
How is gas collected in the gas jar?
Name the substance used for drying ammonia gas? Why cannot substances such as conc.H2SO4, anhydrous calcium chloride and phosphorus pentoxide be used for drying ammonia gas?
Name the following
Gas obtained by treating chlorine in excess of ammonia.
Name the following
Solid obtained by passing ammonia over heated copper oxide
Name the following
Products obtained by burning ammonia in oxygen.
Name the following
Substance used to dry ammonia
Name the following
Liquid when added to metallic nitride that yield ammonia.
Name the following
Indicator that turns deep pink when treated with NH4OH.
Name the following
Products obtained by treating with excess chlorine.
Name the following
Basic gas that is used as a refrigerant.
Name the following
Solution used to remove fat grease.
Name the following
Salt known as ammoniac.
Name the following
Salt used to clean metal surface before soldering, tinning, etc.
Name the following
Ammonium salt used in explosive.
Name the following
An acidic gas which reacts with a basic gas liberating neutral gas.
Name the following
A nitride of divalent metal which reacts with hot water producing ammonia.
Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE 9.1 Ammonia Exercise 2 [Pages 219 - 220]
Name the process used to manufacture ammonia from its elements.
Ammonia is manufactured by Haber Process.
Under what conditions do the reactants combine to form ammonia? Give a balanced equation for the reaction.
Ammonia is manufactured by Haber Process.
In what ratio by volume, are the above reactants used.
State one possible source of each reactant used in the process.
What is the function of:
(i) finely divided iron
(ii) Molybdenum in the above process
Mention two possible ways by which ammonia produced is removed from unchanged gases.
How will you demonstrate the solubility of ammonia in water? Explain.
Write the equation for the action of heat on:
(i) Ammonium chloride
(ii) Ammonium nitrate
State whether each reaction is an example of thermal dissociation or thermal decomposition.
List the properties of ammonia that make it
(i) A good refrigerant
(ii) A cleaning agent
(iii) As a source of hydrogen
Give three uses of ammonium chloride.
Why is ammonium hydroxide used in qualitative analysis? Give two equations to justify your answer.
Name two fertilizers manufactured from ammonia.
Write an equation for the reaction to prepare one fertilizer from ammonia.
Explain the following:
The source of heat is removed after sometime in Haber's process.
Explain the following:
Ammonium nitrate is not used in the laboratory preparation of \[\ce{NH3}\].
Explain the following:
Ca(OH)2 is preferred over NaOH in the preparation of ammonia.
Explain the following:
Liquid NH3 has no action on litmus, while liquor ammonia has an effect.
Explain the following:
Dry N2 and H2 must be used in the Haber's process.
Explain the following:
A promoter is added along with the catalyst in the Haber's process.
Explain the following:
Aqueous ammonia conducts electricity.
Explain the following:
Ammonia cannot be collected over water.
Explain the following:
Ammonia is present in sewage water.
Explain the following:
Ammonia solution is used in the laboratory to identify metalions.
Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE 9.1 Ammonia Exercise 3 [Page 221]
Give balanced reactions for the following conversion:
Ammonia to nitrogen using an acidic gas.
Give balanced reactions for the following conversion:
Ammonia to nitrogen using copper oxide.
Give balanced reactions for the following conversion:
Ammonia to nitrogen using oxygen.
Give balanced reactions for the following conversion:
Ammonia to nitrogen using trichloride.
Give balanced reactions for the following conversion:
Ammonia to ammonium sulphate.
Give balanced reactions for the following conversion:
Ammonia solution to an amphoteric hydroxide.
Give balanced reactions for the following conversion:
Ammonia to ammonium carbonate.
Give balanced reactions for the following conversion:
Copper oxide to copper.
What do you observe when Excess ammonia is mixed with chlorine.
What do you observe when Ammonia in excess is mixed with chlorine.
What do you observe when Filter paper dipped in colourless phenolphthalein is introduced into ammonia.
What do you observe when Ammonia is passed over heated lead oxide.
What do you observe when Ammonium solution is added to ferric chloride solution.
What do you observe when Ammonia solution is added drop by drop and then in excess to aqueous copper sulphate solution.
What do you observe when Ammonia comes in contact with the eyes of a person.
Give balanced equation to prove that ammonia Is alkaline in nature
Give balanced equation to prove that ammonia Has the element hydrogen in it.
Give balanced equation to prove that ammonia Has the element nitrogen in it.
Differentiate between:
Action on indicators or dry ammonia gas and aqueous ammonia
Differentiate between:
Reaction of excess ammonia with chlorine and NH3 with excess chlorine
Differentiate between:
Aqueous ferrous and ferric sulphate solution
Give one use each of four different compounds of ammonia.
Describe two tests to identify ammonia and ammonium ions in an aqueous solution.
Describe all what you will observe and write chemical equation, when limited amount of ammonia gas is passed through the following aqueous solution:
ZnCl2
Describe all what you will observe and write chemical equation, when limited amount of ammonia gas is passed through the following aqueous solution:
FeSO4
Describe all what you will observe and write chemical equation, when limited amount of ammonia gas is passed through the following aqueous solution:
FeCl3
Describe all what you will observe and write chemical equation, when limited amount of ammonia gas is passed through the following aqueous solution:
Pb(NO3)2
Describe all what you will observe and write chemical equation, when limited amount of ammonia gas is passed through the following aqueous solution:
CuSO4
Describe all what you will observe and write chemical equation, when limited amount of ammonia gas is passed through the following aqueous solution:
CrCl3
What are chlorofluorocarbons, why they become popular as substitute of ammonia as refrigerant?
Give two examples of suitable alternatives of CFCs which can be used as refrigerant and are non-ozone deflecting?
Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE 9.1 Ammonia Exercise 4 [Page 222]
Choose the correct answer from the options given below :
Ammonia is produced when ammonium chloride is heated with
Potassium nitrate
Slacked lime
Quick lime
Sodium nitrate
Choose the correct answer from the options given below :
Ammonia reacts with excess chlorine to form
NH4Cl
NH3,NCl3,HCl
N2,NH4Cl
NCl3,HCl
Choose the correct answer from the options given below :
Nessler's reagent is an alkaline solution of
HgI2
K2HgI4
HgNH2I
NH2HgO HgI
Choose the correct answer from the options given below :
Ammonia is commercially prepared by
Haber's process
Ostwald process
Contact process
Head chamber process
Choose the correct answer from the options given below :
The other products obtained along with water where NH3 is passed over heated CuO are
Cu(NH2)2 and H2
[Cu(NH3)4]2+ and O2
Cu and N2
None of these
Choose the correct answer from the options given below :
Liquid ammonia is used in refrigeration because of its
High dipole moment
Baricity
Stability
High heat of vaporization
Choose the correct answer from the options given below :
In the Haber's process for the manufacture of NH3, iron is used as catalyst and molybedenum as promoter. The function of promoter is
To Increase the rate of combination of gases
To raise the activation energy of the reaction
To increase the activity of the catalyst
To increase the yield of ammonia.
Pick the odd number out from the list, giving reasons for your answer:
Ammonia, sulphur dioxide, hydrogen chloride, carbon dioxide
Complete and balance the equation: CuO(heated) + NH3 →
Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE 9.1 Ammonia Exercise 5 [Page 223]
Copy and complete the following table :
| Name of the products | Name of the catalyst | Approximate ternperature | Approximate pressure | |
| Haber's Process |
Copy and complete the following equations :
(i) Mg3N2 + 6H2O →
(ii) 2NH3 + 3CuO →
(iii) 8NH3 + 3Cl2 →
(iv) 4NH3 + 5O2 →
Choose the correct word or phrase from the bracket to complete the following sentence:
Heating ammonium chloride with sodium hydroxide produces ______.
ammonia
nitrogen
Write equation for the following:
Burning of ammonia in oxygen.
Write equation for the following:
Catalytic oxidation of ammonia
(a) Name the catalyst used in 1(ii)
(b) In the reaction referred to in 1(ii), the catalyst glows red hot. Why?
(c) What is the name of the industrial process which starts with the reaction reffered to in 1 (ii)?
Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE 9.1 Ammonia Exercise 6 [Page 224]
How is ammonia soluble in water?
Give two reasons to show that the solution of ammonia in water contains hydroxylions.
Name a simple method you would employ to prepare ammonium salts in your laboratory.
Look at the following reaction :
A. Nitrogen + metal → compound X
B. X + water → ammonia + another compound
C. Ammonia + metal oxide → metal + water + N2
(i) One metal that can be used for reaction in A is magnesium.
(a) Write the formula of the compound X, that is formed.
(b) Write the balanced equation for reaction B, where Xis the compound formed.
( c) Which property of ammonia is demonstrated by reaction C?
Industrially, ammonia is obtained by direct combination of nitrogen and hydrogen.
(i) Write balanced equation for the direct combination of nitrogen and hydrogen.
(ii) Which of the metals - iron, platinum, copper - catalyse this direct combination?
(iii) Is the formation of ammonia promoted by high pressure or low pressure?
Choose the correct word or phrase from the bracket to complete the following sentence.
Heating ammonium chloride with sodium hydroxide produces ______
ammonia
nitrogen
Choose the correct word or phrase from the bracket to complete the following sentences.
Heating solution of ammonium chloride with sodium nitrite produces ______
ammonia
nitrogen
Write the equation for:
The preparation of ammonia from ammonium chloride and calcium hydroxide.
Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE 9.1 Ammonia Exercise 7 [Page 225]
Write the equation for the formation of ammonia by the action of water on magnesium nitride.
Why is ammonia not collected over water?
Write the equation for the reaction in the Haber process, that forms ammonia.
State the purpose of liquefying ammonia produced in the process.
Which feature of the ammonia molecule leads to the formation of the ammonium ion when ammonia dissolves in water?
Name the other ion formed when ammonia dissolves in water.
Give one test that can be used to detect the presence of the ion produced in (i) and (ii).
Write the equations for the following reactions, which result in the formation of ammonia:
(i) A mixture of ammonium chloride and slaked lime is heated.
(ii) Aluminium nitride and water.
Name the substance used for drying ammonia.
Write an equation to illustrate the reducing nature of ammonia.
With reference to Haber's process for the preparation of ammonia, write the equation and the conditions required.
Write balanced chemical equations for a reaction in which ammonia is oxidised by the following:
(i) A metal oxide
(ii) A gas which is not oxygen
Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE 9.1 Ammonia Exercise 8 [Page 226]
Choose the correct answer:
Ammonia can be obtained by adding water to ______.
Ammonium chloride
Ammonium nitrite
Magnesium nitride
Magnesium nitrate
Write the equation for the following :
Ammonium chloride is treated with sodium hydroxide.
State your observation for the following cases
(i) Ammonia gas is burnt in an atomosphere of oxygen in the absence of a catalyst
(ii) Glass rod dipped in ammonium hydroxide is brought near the mouth of the concentrated hydrochloric acid bottle
The questions below are related to the manufacture of ammonia.
(i) Name the process
(ii) In what ratio must the reactants be taken
(iii) Name the catalyst used
(iv) Give the equation for the manufacture of ammonia
(v) Ammonia can act as a reducing agent write a relevant equation for such a reaction.
The diagram shows a simple arrangement of the fountain experiment:
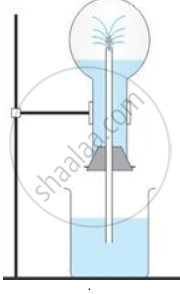
(i) Name the two gases you have studied which can be used in this experiment
(ii) What is the common properly demonstrated by this experiment
Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE 9.1 Ammonia Exercise 9 [Page 227]
The diagram shows an experimental set up for the laboratory preparation of a pungent smelling gas. The gas is alkaline in nature.

- Nature the gas collected in the jar.
- Write the balance equation for the above preparation.
- How is the gas being collected?
- Name the drying agent used.
- How will you find that the jar is full of gas?
Copy and complete the following table relating to important industrial process:
| Name of the process | Temperature | Catalyst | Equation for the catalyzed reaction |
| Haber's Process |
Give a balanced equation for the reduction of hot copper (II) oxide to copper using ammonia gas.
Give balanced equation of :
Action of heat an a mixture of copper an nitric acid
Name the other ion formed when ammonia dissolves in water
Given one test can be used to detect the presence of the ion produced
Give balanced chemical equations for each of the following:
Lab preparation of ammonia using an ammonium salt
Give balanced chemical equations for the following:
Reaction of ammonia with excess chlorine
Give balanced chemical equations for the following:
Reaction of ammonia with sulphuric acid.
Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE 9.1 Ammonia Exercise 10 [Page 228]
Name the gas evolved when the following mixtures are heated:
Calcium hydroxide and ammonium chloride
Name the gas evolved when the following mixtures are heated:
Sodium nitrate and ammonium chloride.
Write balanced chemical equation for the following:
When excess of ammonia is treated with chlorine.
Write balanced chemical equations for the following:
An equation to illustrate the reducing nature of ammonia.
Identify the substance underlined, in the following case:
Cation that does not form a precipitate with ammonium hydroxide but forms one with sodium hydroxide.
Identify the substance underlined, in the following case:
A solid formed by reaction of two gaes, one of which is acidic and the other basic in nature.
Write a balanced chemical equation for reaction of ammonia with heated copper oxide
Laboratory preparation of ammonia from aammonia chloride
Catalyst oxidation of ammonia.
State one relevant observation of burning of ammonia in air.
Certain blank spaces are left in the following table and these are labelled as A,B,C,Da and E. Identify each of then
|
1 |
HCl gas |
NaCl + H2SO4 |
A = ________ |
Conc.H2SO4 |
B = ___ |
|
2 |
NH3 gas |
C = ____ |
Mg(OH)2NH3 |
D = ______ |
E = ___ |
Solutions for 9.1: Ammonia
![Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE chapter 9.1 - Ammonia Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE chapter 9.1 - Ammonia - Shaalaa.com](/images/chemistry-part-2-english-class-10-icse_6:083608507a004841af01e3c142179570.PNG)
Frank solutions for Chemistry - Part 2 [English] Class 10 ICSE chapter 9.1 - Ammonia
Shaalaa.com has the CISCE Mathematics Chemistry - Part 2 [English] Class 10 ICSE CISCE solutions in a manner that help students grasp basic concepts better and faster. The detailed, step-by-step solutions will help you understand the concepts better and clarify any confusion. Frank solutions for Mathematics Chemistry - Part 2 [English] Class 10 ICSE CISCE 9.1 (Ammonia) include all questions with answers and detailed explanations. This will clear students' doubts about questions and improve their application skills while preparing for board exams.
Further, we at Shaalaa.com provide such solutions so students can prepare for written exams. Frank textbook solutions can be a core help for self-study and provide excellent self-help guidance for students.
Concepts covered in Chemistry - Part 2 [English] Class 10 ICSE chapter 9.1 Ammonia are Ammonia, Physical Properties of Ammonia, Chemical Properties of Ammonia, Uses of Ammonia, Laboratory Preparation of Ammonia Gas, Preparation of Aqueous Ammonia, Manufacture of Ammonia (Haber's Process), General Methods of Preparation of Ammonia Gas, Tests for Ammonia Gas and Ammonium Ion.
Using Frank Chemistry - Part 2 [English] Class 10 ICSE solutions Ammonia exercise by students is an easy way to prepare for the exams, as they involve solutions arranged chapter-wise and also page-wise. The questions involved in Frank Solutions are essential questions that can be asked in the final exam. Maximum CISCE Chemistry - Part 2 [English] Class 10 ICSE students prefer Frank Textbook Solutions to score more in exams.
Get the free view of Chapter 9.1, Ammonia Chemistry - Part 2 [English] Class 10 ICSE additional questions for Mathematics Chemistry - Part 2 [English] Class 10 ICSE CISCE, and you can use Shaalaa.com to keep it handy for your exam preparation.
