Advertisements
Advertisements
Question
What do you observe when Excess ammonia is mixed with chlorine.
Solution
When excess ammonia is mixed with chlorine, ammonium chloride and
nitrogen is formed.
\[\ce{8NH3(excess) + 3CI2 -> N2 + 6NH4CI}\]
APPEARS IN
RELATED QUESTIONS
Ammonium salts decompose on heating. What other property do ammonium salts have in common?
What is liquor ammonia fortis?
Choose the correct word or phrase from the bracket to complete the following sentences.
Heating solution of ammonium chloride with sodium nitrite produces ______
The diagram shows a simple arrangement of the fountain experiment:
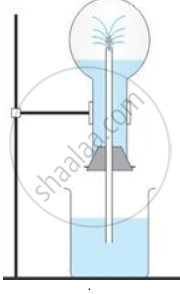
(i) Name the two gases you have studied which can be used in this experiment
(ii) What is the common properly demonstrated by this experiment
Name the gas evolved when the following mixtures are heated:
Calcium hydroxide and ammonium chloride
Write an equation to illustrate the reducing nature of ammonia.
Of the two gases, ammonia and hydrogen chloride, which is denser? Name the method of collection of this gas.
State a relevant reason for the following:
Ammonia gas is not collected over water.
