Advertisements
Advertisements
Question
What do you observe when Ammonium solution is added to ferric chloride solution.
Solution
On adding ammonium solution to ferric chloride solution we get
ammonium chloride and reddish brown precipi tate of ferric hydroxide
which is insoluble in excess of ammonium solution .
\[\ce{FeCI3 + 3NH4OH -> 3NH4CI + Fe(OH)3}\]
APPEARS IN
RELATED QUESTIONS
State what you observe when a piece of moist red litmus paper is placed in a gas jar of ammonia.
Name a metallic chloride soluble in ammonium hydroxide.
State your observation for the following cases
(i) Ammonia gas is burnt in an atomosphere of oxygen in the absence of a catalyst
(ii) Glass rod dipped in ammonium hydroxide is brought near the mouth of the concentrated hydrochloric acid bottle
The diagram shows a simple arrangement of the fountain experiment:
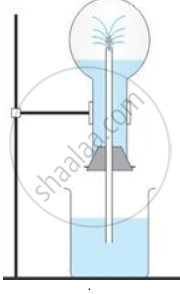
(i) Name the two gases you have studied which can be used in this experiment
(ii) What is the common properly demonstrated by this experiment
Name the gas evolved when the following mixtures are heated:
Calcium hydroxide and ammonium chloride
Name the gas evolved when the following mixtures are heated:
Sodium nitrate and ammonium chloride.
Given one test can be used to detect the presence of the ion produced
What do you observe when Filter paper dipped in colourless phenolphthalein is introduced into ammonia.
Differentiate between:
Action on indicators or dry ammonia gas and aqueous ammonia
