Advertisements
Advertisements
Question
Name the gas evolved when dilute hydrochloric acid is added to: Zinc Metal
Solution
Hydrogen
APPEARS IN
RELATED QUESTIONS
Hydrochloric acid contains (i) hydrogen (ii) chlorine. Prove it. Write equations for the reactions.
Name the following: A greenish yellow gas.
Give a balanced equation when dilute hydrochloric acid is added to : Zinc Metal
Name the gas evolved when dilute hydrochloric acid is added to: Lead (II) sulphide
A solution of hydrogen chloride in water is prepared. The following substances are added to separate portions of the solution:
| S. No. | Substances added | Gas evolved | Odour |
| 1. | Calcium carbonate | _________ | _________ |
| 2. | Magnesium ribbon | _________ | _________ |
| 3. | Manganese (IV) oxide with heating | _________ | _________ |
| 4. | Sodium sulphide | _________ | _________ |
Complete and balance the following reaction, state whether dilute or conc. acid is used.
\[\ce{NH4OH + HCl->}\]
Complete and balance the following reaction, state whether dilute or conc. acid is used.
\[\ce{NH4OH + HCl->}\]
Complete and balance the following reaction, state whether dilute or cone. acid is used.
\[\ce{NA4OH + HCl -> }\]
Study the flow chart and give balanced equations with conditions for the conversions A, B, C, D and E.
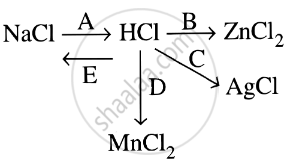
Study the flow chart and give balanced equations with conditions for the conversions A, B, C, D and E.