Advertisements
Advertisements
Question
Name the gas evolved when dilute hydrochloric acid is added to: Magnesium bicarbonate
Solution
Carbon dioxide
APPEARS IN
RELATED QUESTIONS
Name a black metallic oxide which reacts with hydrochloric acid to give a coloured solution.
Name the experiment which demonstrates that hydrogen chloride is soluble in water.
Distinguish between the following pairs of compounds using the reagent given in the bracket.
Mangenese dioxide and copper (II) oxide. (using concentrated HCl)
Give a balanced equation when dilute hydrochloric acid is added to : Potassium bisulphite
Convert Hydrochloric acid to nascent chlorine.
Complete and balance the following reaction, state whether dilute or cone. acid is used.
\[\ce{NA4OH + HCl -> }\]
Study the flow chart and give balanced equations with conditions for the conversions A, B, C, D and E.
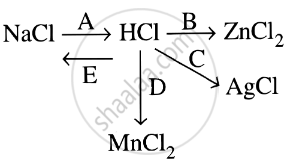
Complete and balance the following reaction, state whether dilutes or conc. acid is used.
\[\ce{NH4OH + HCl -> }\]
Complete and balance the following reaction, state whether dilute or conc. acid is used.
\[\ce{NH4OH + HCl ->}\]
Study the flow chart and give balanced equations with conditions for the conversions A, B, C, D and E.

