Advertisements
Advertisements
Question
Name the gas evolved when dilute hydrochloric acid is added to: Potassium bisulphite
Solution
sulphur dioxide
APPEARS IN
RELATED QUESTIONS
Name a chloride which is solube in excess of ammonium hydroxide
Give reason for the following:
silver nitrate solution can be used to distinguish HCl from HNO3
Complete and balance the following reaction, state whether dilute or conc. acid is used.
\[\ce{NH4OH + HCl -> \underline{\phantom{..........}}}\].
Study the flow chart and give balanced equations with conditions for the conversions A, B, C, D and E.
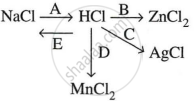
Distinguish between the following pairs of compounds using the reagent given in the bracket.
Mangenese dioxide and copper (II) oxide. (using concentrated HCl)
Name the following :
Acid present in the stomach of mammals.
Calcium oxide and phosphorous pentoxide are very good drying agents but they are not used to dry HCI gas. Give reasons for each.
Choose the correct answer from the options given below:
Which of the following statement is not correct ?
How will you prove that hydrochloric acid contains
- hydrogen
- chlorine?
Write equations for the reactions.
Complete and balance the following reaction, state whether dilute or cone. acid is used.
\[\ce{NH4OH + HCl ->}\]
