Advertisements
Advertisements
Question
Study the flow chart and give balanced equations with conditions for the conversions A, B, C, D and E.
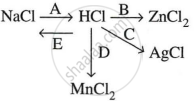
Solution
A: \[\ce{NaCl + H2SO4 ->[< 200°C] NaHSO4 + HCl ^}\]
B: \[\ce{Zn + 2HCl -> ZnCl2 + H2}\]
C: \[\ce{AgNO3 + HCl -> AgCl + HNO3}\]
D: \[\ce{MnO2 + 4HCl ->[\Delta] MnCl2 + 2H2O + Cl2 ^}\]
E: \[\ce{HCl + NaOH -> NaCl + H2O}\]
APPEARS IN
RELATED QUESTIONS
Name two gases which chemically combine to form a liquid.
Name the chemical in which gold can be dissolved.
Give a balanced equation when dilute hydrochloric acid is added to : Zinc Metal
Give a balanced equation when dilute hydrochloric acid is added to : Sodium sulphite
Give a balanced equation when dilute hydrochloric acid is added to : Potassium bisulphite
How will you identify?
Chlorine gas
Convert Hydrochloric acid to nascent chlorine.
Complete and balance the following reaction, state whether dilute or cone. acid is used.
\[\ce{NH4OH + HCl ->}\]
Complete and balance the following reaction, state whether dilute or conc. acid is used.
NH4OH + HCl ⟶
Study the flow chart and give balanced equations with conditions for the conversions A, B, C, D and E.

