Advertisements
Advertisements
Question
Write the equation for :
The preparation of hydrogen chloride from sodium chloride and sulphuric acid. State whether the sulphuric acid should be concentrated or dilute.
Solution
\[\ce{\underset{\text{Sodium chloride}}{NaCl} + \underset{\text{Sulphuric acid(Conc.)}}{H2SO4} ->[<200°C] \underset{\text{Sodium hydrogen sulphate}}{NaHSO4} + \underset{\text{Hydrogen chloride gas}}{HCl}}\]
APPEARS IN
RELATED QUESTIONS
Certain blank spaces are left in the following table and these are labelled as A, B, C, D
and E. Identify each of them
| Lab preparation of | Reactants used | Products formed | Drying Agent | Method of collection |
|
| 1 | HCl gas | NaCl + H2SO4 |  |
conc. H2SO4 | 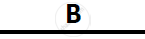 |
| 2 | NH3 gas |
|
Mg(OH)2 NH3 |
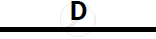 |
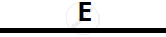 |
The following are pertaining to the laboratory preparation of hydrogen chloride gas.
Write the equation for its preparation mentioning the condition required.
The following are pertaining to the laboratory preparation of hydrogen chloride gas.
Name the drying agent used and justify your choice.
Give the balanced equation for the laboratory preparation of hydrogen chloride gas reaction.
Explain why a solution of hydrogen chloride in water turns blue litmus red and conducts electricity, while a solution of the same gas in toluene:
- has no effect on litmus, and
- does not conduct electricity.
Name the gas produced when chlorine water is exposed to sunlight.
The drying agent used to dry \[\ce{HCl}\] gas is ______.
Draw a labelled diagram and explain the laboratory preparation of hydrogen chioride gas.
How will you prove that the gas prepared is HCI?
What are the important precautions?
Explain, why (or give reasons for)
HCI gas does not conduct electricity, but hydrochloric acid conducts electricity.
Describe an experiment to prove the following:
HCI gas contains the element chlorine.
Mention the reaction condition and give balanced equation to obtain: Cl2 gas from HCI acid.
Complete and balance the equations:
KMnO4 + HCI → _______+ _______ + _______ + ______ + 8H2O
Explain, why the following statement is not correct:
Lead chloride can be prepared by adding dilute HCI to lead sulphate solution.
What property of hydrogen chloride is demonstrated when it is collected by downward delivery (upward displacement) ?
The following question is pertaining to the laboratory pertaining hydrogen chloride gas.
Name the drying agent used and justify your choice.
One chemical test that would enable you to distinguish between the following pair of chemicals. Describe what happens with each chemical or state 'no visible reaction'.
Sodium chloride solution and sodium nitrate solution.
Answer the following question related to the laboratory preparation of the hydrogen chloride gas:
Why is sodium chloride preferred to other metallic chlorides?
Complete and balance the following reaction, state whether dilute or conc. acid is used.
\[\ce{NH4OH + HCl ->}\]
