Advertisements
Advertisements
Question
The following are pertaining to the laboratory preparation of hydrogen chloride gas.
Write the equation for its preparation mentioning the condition required.
Solution
The equation for the laboratory preparation of hydrogen chloride gas:

Although it is a reversible reaction, it goes to completion as hydrogen chloride continuously escapes as a gas.
The reaction can occur up to the stage of the formation of sodium sulphate on heating above 200°C.
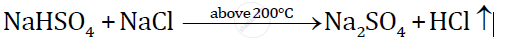
APPEARS IN
RELATED QUESTIONS
Identify the gas evolved and give the chemical test in the following cases
Dilute hydrochloric acid reacts with iron (II) sulphide.
The following are pertaining to the laboratory preparation of hydrogen chloride gas.
State a safety precaution you would take during the preparation of hydrochloric acid.
State your observation in given case When dilute hydrochloric acid is added to sodium carbonate crystals
Name the acid used for the preparation of hydrogen chloride gas in the laboratory. Why is this particular acid preferred to other acids?
Draw a labelled diagram for the laboratory preparation of hydrogen chloride gas and answer the following.
- Name the acid used. Why is this particular acid preferred to other acids?
- Give the balanced equation for the reaction.
- Name the drying agent used in drying hydrogen chloride gas.
- Phosphorous pentoxide and calcium oxide are good drying agents, but they cannot be used to dry hydrogen chloride gas. Why?
- Why is the direct absorption of \[\ce{HCl}\] gas in water not feasible?
- What arrangement is done to dissolve \[\ce{HCl}\] gas in the water?
Name the drying agents phosphorus pentoxide and calcium oxide are good drying agent but they cannot be used to dry hydrogen chloride gas. Why?
Explain why thick white fumes are formed when a glass rod dipped in \[\ce{NH4OH}\] is brought near the mouth of a bottle full of \[\ce{HCl}\] gas.
Write the main difference in hydrogen chloride gas and hydrochloric acid.
The drying agent used to dry \[\ce{HCl}\] gas is ______.
Explain, why (or give reasons for)
In the preparation chloride from sodium chloride, the gas can be obtained below 200°C or above. But the lower temperature is preferred.
Describe an experiment to prove the following:
HCI gas contains the element chlorine.
Mention the reaction condition and give balanced equation to obtain: Cl2 gas from HCI gas.
Write balanced equation for the reaction between dilute hydrochloric acid and sodium sulphite.
Write the equation for:
The reaction between hydrogen chloride and ammonia.
The following question is pertaining to the laboratory pertaining hydrogen chloride gas.
Name the drying agent used and justify your choice.
One chemical test that would enable you to distinguish between the following pair of chemicals. Describe what happens with each chemical or state 'no visible reaction'.
Sodium chloride solution and sodium nitrate solution.
In the laboratory preparation, HCl gas is dried by passing through ______.
State a relevant reason for the following:
Hydrogen chloride gas cannot be dried over quick lime.
Complete and balance the following reaction, state whether dilute or conc. acid is used.
\[\ce{NH4OH + HCl ->}\]
