(English Medium)
Academic Year: 2014-2015
Date: March 2015
Advertisements
Question 1 is compulsory.
Attempt any four From Question 2 to Question 7
Select from the list the gas that matches the description given in case
[ammonia, ethane, hydrogen chloride, hydrogen sulfide, ethyne]
This gas is used as a reducing agent in reducing copper oxide to copper
Chapter: [0.08199999999999999] Ammonia
Select from the list the gas that matches the description given in case
[ammonia, ethane, hydrogen chloride, hydrogen sulfide, ethyne]
This gas produces dense white fumes with ammonia gas.
Chapter: [0.081] Hydrogen Chloride
Select from the list the gas that matches the description given in case
[ammonia, ethane, hydrogen chloride, hydrogen sulphide, ethyne]
This gas is used for welding purposes.
Chapter: [0.09] Organic Chemistry
Select from the list the gas that matches the description given in case
[ammonia, ethane, hydrogen chloride, hydrogen sulfide, ethyne]
This gas is also a saturated hydrocarbon
Chapter: [0.09] Organic Chemistry
Select from the list the gas that matches the description given in case
[ammonia, ethane, hydrogen chloride, hydrogen sulfide, ethyne]
This gas has a characteristic rotten egg smell.
Chapter: [0.081] Hydrogen Chloride
Choose the most appropriate answer of the following:
Among the elements given below, the element with the least electronegativity is:
(A) Lithium
(B) Carbon
(C) Boron
(D) Fluorine
Chapter: [0.01] Periodic Table, Periodic Properties and Variations of Properties
Choose the most appropriate answer of the following:
Identify the statement which does not describe the property of alkenes:
(A) They are unsaturated hydrocarbons
(B) They decolorize bromine water
(C) They can undergo addition as well as substitution reactions
(D) They undergo combustion with oxygen forming carbon dioxide and water.
Chapter: [0.09] Organic Chemistry
Choose the most appropriate answer of the following:
This is not an alloy of copper:
(A) Brass
(B) Bronze
(C) Solder
(D) Duralumin.
Chapter: [0.07] Metallurgy
Choose the most appropriate answer of the following:
Bonding in this molecule can be understood to involve coordinate bonding
(A) Carbon tetrachloride
(B) Hydrogen
(C) Hydrogen chloride
(D) Ammonium chloride
Chapter: [0.02] Chemical Bonding
Choose the most appropriate answer of the following:
Which of the following would weigh the least?
(A) 2-gram atoms of Nitrogen.
(B) 1mole of Silver
(C) 22.4 liters of oxygen gas at 1 atmospheric pressure and 273K
(D) 6.02 x 1023 atoms of carbon.
[Atomic masses: Ag=108, N=14, O=16, C=12]
Chapter: [0.01] Periodic Table, Periodic Properties and Variations of Properties
Calculate the mass of Calcium that will contain the same number of the atom as are present in 3.2 gm of Sulphur.
[Atomic masses: S=32, Ca=40]
Chapter: [0.01] Periodic Table, Periodic Properties and Variations of Properties
If 6 liters of hydrogen and 4 liters of chlorine are mixed and exploded and if water is added to the gases formed, find the volume of the residual gas.
Chapter: [0.05] Mole Concept and Stoichiometry
If the empirical formula of a compound is CH and it has a vapor density of 13, find the molecular formula of the compound.
Chapter: [0.05] Mole Concept and Stoichiometry
State one relevant observation for the following:
When crystals of copper nitrate are heated in a test tube.
Chapter: [0.083] Nitric Acid
State one relevant observation for the following
When the gaseous product obtained by dehydration of ethyl alcohol is passed through bromine water.
Chapter: [0.09] Organic Chemistry
State one relevant observation for the following
When hydrogen sulfide gas is passed through lead acetate solution.
Chapter: [0.081] Hydrogen Chloride
State one relevant observation for the following
When ammonia gas is burnt in an atmosphere of excess oxygen.
Chapter: [0.08199999999999999] Ammonia
State one relevant observation for the following:
At the anode when aqueous copper sulphate solution is electrolysed using copper electrodes.
Chapter: [0.06] Electrolysis
The acid which is used in the preparation of a volatile acid
Chapter: [0.084] Sulphuric Acid
Identify the acid in each case:
The acid which produces sugar charcoal from sugar
Chapter: [0.084] Sulphuric Acid
The acid which is prepared by catalytic oxidation of ammonia.
Chapter: [0.08199999999999999] Ammonia
Identify the acid in the case:
The acid on mixing with lead nitrate solution produces a white precipitate, which is insoluble even on heating.
Chapter: [0.084] Sulphuric Acid
The acid on mixing with silver nitrate solution produces a white precipitate which is soluble in excess ammonium hydroxide
Chapter: [0.081] Hydrogen Chloride
Give appropriate scientific reasons for Zinc oxide can be reduced to zinc metal by using
carbon, but aluminium oxide cannot be reduced by a reducing agent
Chapter: [0.07] Metallurgy
Give appropriate scientific reasons for Carbon tetrachloride does not conduct electricity.
Chapter: [0.02] Chemical Bonding
Give appropriate scientific reasons for During electrolysis of molten lead bromide, graphite anode is preferred to other electrodes.
Chapter: [0.06] Electrolysis
Give appropriate scientific reasons for The electrical conductivity of acetic acid is less in comparison to the electrical conductivity of dilute sulphuric acid at a given concentration.
Chapter: [0.084] Sulphuric Acid
Give appropriate scientific reasons for Electrolysis is of molten lead bromide is considered to be a redox reaction.
Chapter: [0.06] Electrolysis
Give balanced chemical equations for the following conversions A, B, and C:
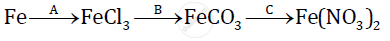
Chapter: [0.05] Mole Concept and Stoichiometry
Differentiate between the terms strong electrolyte and weak electrolyte. (stating any two differences)
Chapter: [0.06] Electrolysis
Explain the bonding in methane molecule using electron dot structure.
Chapter: [0.02] Chemical Bonding
Advertisements
The metal of Group 2 from top to bottom arc Be, Mg, Ca, Sr, and Ba.
1) Which one of these elements will form ions most readily and why?
2) State the common feature in the electronic configuration of all these elements.
Chapter: [0.01] Periodic Table, Periodic Properties and Variations of Properties
Arrange the following as per the instruction given in the bracket.
Cs, Na, Li, K, Rb (increasing order of metallic character).
Chapter: [0.01] Periodic Table, Periodic Properties and Variations of Properties
Arrange the following as per the instruction given in the bracket:
Mg, Cl, Na, S, Si (decreasing order of atomic size).
Chapter: [0.01] Periodic Table, Periodic Properties and Variations of Properties
Arrange the following as per the instructions given in the brackets:
Na, K, Cl, S, Si (increasing order ionization energy)
Chapter: [0.01] Periodic Table, Periodic Properties and Variations of Properties
Arrange the following as per the instructions given in the brackets:
Cl, F, Br, I (increasing order of electron affinity)
Chapter: [0.01] Periodic Table, Periodic Properties and Variations of Properties
Choose the most appropriate answer from the following list of oxides which fit the description.
[SO2, SiO2, Al2O3, MgO, CO, Na2O]
A basic oxide.
Chapter: [0.04] Analytical Chemistry
Choose the most appropriate answer from the following list of oxides which fit the description
[SO2, SiO2, Al2O3, MgO, CO, Na2O]
An oxide which dissolves in water forming an acid
Chapter: [0.04] Analytical Chemistry
Choose the most appropriate answer from the following list of oxides which fit the description
[SO2, SiO2, Al2O3, MgO, CO, Na2O]
An amphoteric oxide
Chapter: [0.04] Analytical Chemistry
Choose the most appropriate answer from the following list of oxides which fit the description.
A covalent oxide of a metalloid.
SO2
SiO2
Al2O3
MgO
CO
Na2O
Chapter: [0.02] Chemical Bonding
Element X is a metal with a valency 2, Y is 3 non- metal with a valency 3.
1) Write an equation to show how Y from an ion.
2) If Y is a diatomic gas, write an equation for the direct combination of X and Y to form a compound.
Chapter: [0.01] Periodic Table, Periodic Properties and Variations of Properties
Give balanced chemical equations for the ethanoic acid to ethyl ethanoate.
Chapter: [0.09] Organic Chemistry
Give balanced chemical equations for Calcium carbide to ethyne.
Chapter: [0.09] Organic Chemistry
Give balanced chemical equations for Sodium ethanoate to methane.
Chapter: [0.09] Organic Chemistry
Using their structural formulae identify the functional group by circling them
Dimethyl ether
Chapter: [0.09] Organic Chemistry
Using their structural formulae identify the functional group by circling them
Propanone.
Chapter:
Name the following:
The process by which ethane is obtained from ethane.
Chapter: [0.09] Organic Chemistry
Name the following:
A hydrocarbon which contributes towards the greenhouse effect.
Chapter: [0.09] Organic Chemistry
Name the following:
The distinctive reaction that takes place when ethanol is treated with acetic acid.
Chapter: [0.09] Organic Chemistry
Name the following:
The property of element by virtue of which atoms of the element can link to each other in the form of a long chain or ring structure.
Chapter: [0.02] Chemical Bonding
Name the following:
Reaction when an alkyl halide is treated with alcoholic potassium hydroxide.
Chapter: [0.09] Organic Chemistry
Identify the anion present in the following compounds:
A salt M on treatment with concentrated sulphuric acid produces a gas which fumes in moist air and gives dense fumes with ammonia
Chapter: [0.02] Chemical Bonding
Identify the anion present in the following compounds:
A salt D on treatment with dilute sulphuric acid produces a gas which turns lime water milky but has no effect on acidified potassium dichromate solution
Chapter: [0.03] Study of Acids, Bases and Salts
Identify the anion present in the following compounds:
When barium chloride solution is added to salt solution E a white precipitate insoluble in dilute hydrochloric acid is obtained.
Chapter: [0.03] Study of Acids, Bases and Salts
The following table shows the test a student performed on four different aqueous solutions which are X, Y, Z and W. Based on the observations provided, identify the cation present:
| Chemical test | Observation | Conclusion |
| To solution X, ammonium hydroxide is added in minimum quantity first and then in excess |
A dirty white precipitate is formed which dissolves in excess to form a clear solution |
(1) |
| To solution Y ammonium hydroxide is added in the minimum quantity first and then in excess |
A pale blue precipitate is formed which dissolves in excess to form a clear inky blue solution. |
(2) |
| To solution W a small quantity of sodium hydroxide solution is added and then in excess. |
A white precipitate is formed which remains insoluble. |
(3) |
| To a salt Z calcium hydroxide the solution is added and then heated. |
A pungent smelling gas turning moist red litmus paper blue is obtained. |
(4) |
Chapter: [0.02] Chemical Bonding
Give balanced chemical equations for Lab preparation of ammonia using an ammonium salt
Chapter: [0.08199999999999999] Ammonia [0.1] Practical Work
Give balanced chemical equations for Reaction of ammonia with excess chlorine.
Chapter: [0.08199999999999999] Ammonia
Give balanced chemical equations for Reaction of ammonia with sulphuric acid.
Chapter: [0.08199999999999999] Ammonia
Consider the following reaction and based on the reaction answer the questions that follow:

Calculate:
1) the quantity in moles of (NH4)2Cr2O7 if 63gm of(NH4)2Cr2O7 is heated.
2) the quantity in moles of nitrogen formed.
3) the volume in liters or dm3 of N2 evolved at S.T.P.
4) the mass in grams of Cr2O3 formed at the same time
(Atomic masses: H=1, Cr= 52, N=14]
Chapter: [0.05] Mole Concept and Stoichiometry
Advertisements
For the substance given below, describe the role played in the extraction of aluminium.
Cryolite
Chapter: [0.07] Metallurgy
For the substance given below, describe the role played in the extraction of aluminium.
Sodium hydroxide
Chapter: [0.07] Metallurgy
For the substance given below, describe the role played in the extraction of aluminium.
Graphite
Chapter: [0.07] Metallurgy
Explain why In the electrolysis of alumina using the Hall Heroult's Process the electrolyte is covered with powdered coke
Chapter: [0.07] Metallurgy
Explain why Iron sheets are coated with zinc during galvanization.
Chapter: [0.07] Metallurgy
Give balanced chemical equations for the action of sulphuric acid on the following:
Potassium hydrogen carbonate.
Chapter: [0.084] Sulphuric Acid
Give balanced chemical equations for the action of sulphuric acid on the following:
Sulphur
Chapter: [0.084] Sulphuric Acid
In the contact process for the manufacture of sulphuric acid give the equations for the conversion of sulphur trioxide to sulphuric acid.
Chapter: [0.084] Sulphuric Acid
Copy and complete the following table:
| Anode | Electrolyte | |
| Purification of copper |
Chapter: [0.06] Electrolysis
Write the equation taking place at the anode
Chapter: [0.06] Electrolysis
Explain the following:
Dilute nitric acid is generally considered a typical acid but not so in its reaction with metals.
Chapter: [0.083] Nitric Acid
Explain the following:
Concentrated nitric add appears yellow when it is left standing in a glass bottle.
Chapter: [0.083] Nitric Acid
Explain the following:
An all-glass apparatus is used in the laboratory preparation of nitric acid.
Chapter: [0.083] Nitric Acid
The following are pertaining to the laboratory preparation of hydrogen chloride gas.
Write the equation for its preparation mentioning the condition required.
Chapter: [0.081] Hydrogen Chloride [0.1] Practical Work
The following are pertaining to the laboratory preparation of hydrogen chloride gas.
Name the drying agent used and justify your choice.
Chapter: [0.081] Hydrogen Chloride [0.1] Practical Work
The following are pertaining to the laboratory preparation of hydrogen chloride gas.
State a safety precaution you would take during the preparation of hydrochloric acid.
Chapter: [0.081] Hydrogen Chloride [0.1] Practical Work
An element L consists of molecules.
What type of bonding is present in the particles that make up L?
Chapter: [0.02] Chemical Bonding
An element L consists of molecules.
When L is heated with iron metal, it forms a compound FeL. What chemical term would you use to describe the change undergone by L?
Chapter: [0.07] Metallurgy
From the list of the following salts choose the salt that most appropriately fits the description is given in the following:
[AgCl, MgCl2, NaHSO4, PbCO3, ZnCO3, KNO3, Ca(NO3)2]
A deliquescent salt
Chapter: [0.03] Study of Acids, Bases and Salts
From the list of the following salts choose the salt that most appropriately fits the description is given in the following:
[AgCl, MgCl2, NaHSO4, PbCO3, ZnCO3, KNO3, Ca(NO3)2]
An insoluble chloride.
Chapter: [0.03] Study of Acids, Bases and Salts
From the list of the following salts choose the salt that most appropriately fits the
description is given in the following:
[AgCl, MgCl2, NaHSO4, PbCO3, ZnCO3, KNO3, Ca(NO3)2]
On heating, this salt gives a yellow residue when hot and white when cold
Chapter: [0.03] Study of Acids, Bases and Salts
From the list of the following salts choose the salt that most appropriately fits the
description is given in the following:
[AgCl, MgCl2, NaHSO4, PbCO3, ZnCO3, KNO3, Ca(NO3)2]
On heating this salt, a brown colored gas is evolved.
Chapter: [0.03] Study of Acids, Bases and Salts
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
CISCE previous year question papers ICSE Class 10 Chemistry with solutions 2014 - 2015
Previous year Question paper for CISCE ICSE Class 10 -2015 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Chemistry, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of CISCE ICSE Class 10 .
How CISCE ICSE Class 10 Question Paper solutions Help Students ?
• Question paper solutions for Chemistry will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
