Advertisements
Advertisements
Question
Choose the most appropriate answer of the following:
Among the elements given below, the element with the least electronegativity is:
(A) Lithium
(B) Carbon
(C) Boron
(D) Fluorine
Solution
Lithium
Lithium is an element with the least electronegativity.
APPEARS IN
RELATED QUESTIONS
Arrange the following as per the instruction given in the brackets :
F, Cl, Br (Increasing electronegativity)
Name the elements with highest and lowest electronegativity ?
Study the extract of the Periodic Table given below and answer the questions that follow. Give the alphabet corresponding to the element in question. DO NOT repeat an element. 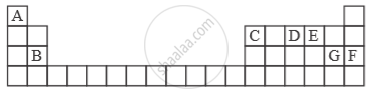
Which element forms electrovalent compound with G?
In terms of electronic configuration, what the elements of a given period and groups have in common?
Fill in the blank
Reactivity ______ down the gruop for alkali metals with increase in electropositive character
The electro negativities (according to Pauling) of the elements in period 3 of the periodic table are as follows.
The elements are arranged in alphabetical order:
Al Cl Mg Na P S Si
1.5 3.0 1.2 0.9 2.1 2.5 1.8
(i) Arrange the elements in the order in which they occur in the periodic table from left to right.
(The group 1 elements first, followed by the group 2 element and so on, up to group 7)
(ii) Choose the word or phrase from the brackets which correctly completes each of the following statements:
(a) The element below sodium in the same group would be expected to have a ______ (lower/higher) electro negativity than sodium and the element above chlorine which would be expected to have a ______ (lower / higher) ionization potential than chlorine.
(b) On moving from left to right in a given period, the number of shells ______ (remains the same / decreases/ increases).
(c) On moving down a group, the number of valence electrons ______ (remains the same / increases/ decreases).
Among the elements given below, the element with the least electronegativity is:
Rewrite the following sentence by using the correct > (greater than) or < (less than) in the blank given :
The electronegativity of iodine is ________ that of chlorine.
Identify the following:
The most electronegative element of Period 3.
In the table below, H does not represent hydrogen. Some elements are given in their own symbol and position in the periodic table while others are shown with a letter.
| IA | IIA | IIIA | IVA | VA | VIA | VIIA | O |
| 1 | 2 | 13 | 14 | 15 | 16 | 17 | 18 |
| Li | D | O | J | Ne | |||
| A | Mg | E | Si | H | K | ||
| A | C | F | G | L |
Select from the table.
- Which is most electronegative.
- How many valence electrons are present in G.
- Write the formula of the compound between B and H.
- In the compound between F and J what type of bond will be formed.
- Draw the electron dot structure for the compound formed between C and K.
