Advertisements
Advertisements
Question
Choose the most appropriate answer from the following list of oxides which fit the description
[SO2, SiO2, Al2O3, MgO, CO, Na2O]
An amphoteric oxide
Solution
Al2O3
APPEARS IN
RELATED QUESTIONS
A compound X (having the vinegar-like smell) when treated with ethanol in the presence of the acid Z, gives a compound Y which has a fruity smell.
The reaction is:
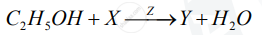
1) Identify Y and Z.
2) Write the structural formula of X.
3) Name the above reaction.
Name: A metallic oxide soluble in excess of caustic soda solution.
Name: A strong alkali
Name: A weak alkali.
Name: Two coloured ions
Name : A yellow monoxide that dissolves in hot and concentrated caustic alkali.
Fill in the blank.
To distinguish soluble salts of zinc and lead ______ [NaOH / NH4OH]can be used.
When ammonium hydroxide is added to solution B, a pale blue precipitate is formed. This pale blue precipitate dissolves in excess ammonium hydroxide to give an inky blue solution. Name the cation present in solution B. What is the probable colour of solution B?
One chemical test that would enable you to distinguish between the following pair of chemicals. Describe what happens with each chemical or state 'no visible reaction'.
Calcium nitrate solution and zinc nitrate solution.
Write balanced equations for a metal that evolves a gas which burns with a pop sound when boiled with alkali solutions.
