Advertisements
Advertisements
Question
Give balanced chemical equations for Lab preparation of ammonia using an ammonium salt
Solution
Ammonia is prepared in the laboratory by using ammonium chloride.
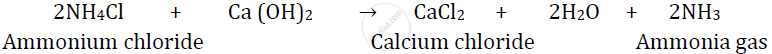
APPEARS IN
RELATED QUESTIONS
Write a balanced chemical equation for the following:
Laboratory preparation of ammonia from ammonium chloride.
Write a balanced chemical equation for the preparation of the following salts
Ammonium sulphate crystals
Name the gas which is prepared by Haber's process.
Laboratory preparation of ammonia from aammonia chloride
How is ammonia gas prepared in laboratory starting from NH4Cl? State the conditions and balanced equation for the preparation.
Name a simple method you would employ to prepare ammonium salts in your laboratory.
A substance 'A' was heated with slaked lime and a gas 'B' with the pungent smell was obtained. Name the substances A and B and give a balanced equation.
Give reasons:
Ammonium nitrate is not used in the preparation of ammonia.
Give reasons:
Conc. \[\ce{H2SO4}\] is a good drying agent, yet it is not used to dry \[\ce{NH3}\].
Ammonia is manufactured by Haber Process.
Under what conditions do the reactants combine to form ammonia? Give a balanced equation for the reaction.
Ammonia is manufactured by Haber Process.
State whether the formation of ammonia is promoted by the use of high pressure or low pressure?
Give the chemical equation(s) to prove that \[\ce{NH3}\] contains nitrogen and hydrogen.
Write observations:
Ammonia gas is passed over heated copper (II) oxide.
State the observation for the following, when:
A glass rod dipped in concentrated HCl acid is brought near ammonia gas.
The following question pertains to the laboratory preparation of Ammonia gas from Magnesium nitride:
Write a balanced chemical equation for its preparation.
The following question pertains to the laboratory preparation of Ammonia gas from Magnesium nitride:
Why is the method seldom used?
