Advertisements
Advertisements
Question
Explain the following:
Dilute nitric acid is generally considered a typical acid but not so in its reaction with metals.
Solution
Dilute nitric acid is generally considered a typical acid but not in its reaction with metals because the action of nitric acid on metals depends on the temperature and concentration of nitric acid. These conditions are not required in case of hydrochloric acid or sulphuric acid
APPEARS IN
RELATED QUESTIONS
Write balanced equations for Action of heat on a mixture of copper and concentrated nitric acid
Give two chemical equations for the following:
Reactions of nitric acid with non-metals
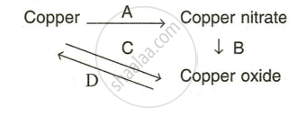
Write equation for the following conversions A, B, C and D.
How will you prepare the following from nitric acid?
Aqua regia
Mention three important uses of nitric acid. Give the property of nitric acid involved in the use.
Explain with the help of a balanced equation, the brown ring test for nitric acid.
What is the purpose of Conc. H2SO4 in the above preparation?
Why is the temperature maintained at 200°C in the above reaction?
Complete and balance the following equation :
HNO3 + FeSO4 + H2SO4 → ______________
Give a balanced equation for the following reaction:
Copper reacts with concentrated nitric acid.
