Advertisements
Advertisements
Question
What is the function of HCI in preparation of aqua-regia?
Solution
HCI dissolves noble metals like gold and platinum. HCI with HNO3 reacts to produce nascent chlorine which reacts with gold, platinum etc. to form their respective chiorides.
APPEARS IN
RELATED QUESTIONS
The acid on mixing with silver nitrate solution produces a white precipitate which is soluble in excess ammonium hydroxide
For the preparation of hydrochloric acid in the laboratory:
What arrangement is done to dissolve hydrogen chloride gas in water?
Give a chemical test to distinguish between the given pairs of chemicals:
Sodium chloride solution and Sodium nitrate solution
State which component is the oxidizing agent in aqua regia.
State the use of aqua-regia.
How will you identify?
An amphoteric hydroxide
Identify the anion present of the following compound:
When barium chloride solution is added to salt solution E a white precipitate insoluble in dilute hydrochloric acid is obtained
State which of the two - a solution of HCl in water or in toluene is an electrolyte. Explain.
State which of the two - a solution of HCl in water or in toluene is an electrolyte. Explain.
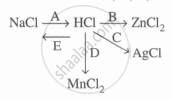
Study the flow chart and give balanced equations with conditions for the conversions A, B, C, D, and E.