Advertisements
Advertisements
Question
Name two gases which combine chemically to form a solid.
Solution
HCI and NH3 combine chemically to form a solid.
APPEARS IN
RELATED QUESTIONS
When dilute HCl is added to a salt Q, a brisk effervescence is produced and the gas turns lime water milky.
When NH4OH solution is added to the above mixture (after adding dilute HCl), it produces a white precipitate which is soluble in excess NH4OH solution.
The aim of the Fountain experiment is to prove that ______.
Study the figure given below and answer the questions which follow:
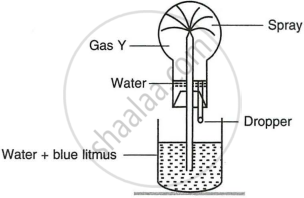
- Identify the gas Y.
- What property of gas Y does this experiment demonstrate?
- Name another gas which has the same property and can be demonstrated through this experiment.
Name :
Two colourless gases which when mixed produce a white solid.
Describe an experiment to prove the following:
HCI gas is heavier than air.
How can you prove that hydrochloric acid contain :
Chlorine?
Choose the correct answer from the options given below: HCl gas can be prepared by direct combination of hydrogen and chlorine gas in presence of
Give reason for the following:
Dilute hydrochloric acid cannot be concentrated by distilling (boiling) the dilute acid.
Dilute hydrochloric acid is added in turn to a mixture of iron and sulphur and to the compound formed between iron and sulphur. Name the gas formed in each case.
The aim of the fountain experiment is to prove that
