Advertisements
Advertisements
Question
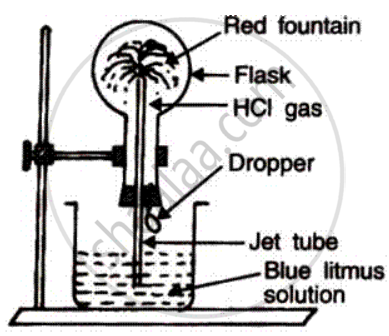
(a) Name the experiment illustrate above.
(b) Which property of hydrogen chloride is demonstrated by this experiment?
(c) State the colour of the water that has entered the round-bottomed flask.
Solution
(a) Fountain experiment.
(b) This experiment demonstrates that hydrogen chloride is very soluble in water.
(c) Red
APPEARS IN
RELATED QUESTIONS
Study the figure given below and answer the questions which follow:
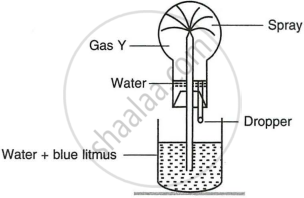
- Identify the gas Y.
- What property of gas Y does this experiment demonstrate?
- Name another gas which has the same property and can be demonstrated through this experiment.
Name :
Two colourless gases which when mixed produce a white solid.
Name two gases which combine chemically to form a solid.
How will you show that hychloric acid contains both hydrogen and chlorine (other than by electrolysis) ?
Give reason for the following:
Dilute hydrochloric acid cannot be concentrated by distilling (boiling) the dilute acid.

The diagram shows an apparatus for the laboratory preparation of hydrogen chloride.
(i) Identify A and B
(ii) Write the equation for the reaction
(iii) How would you check whether or not the gas jar is filled with hydrogen chloride?
(iv) What does the method of collection tell you about the density of hydrogen chloride.
Study the figure given below and answer that questions that follow: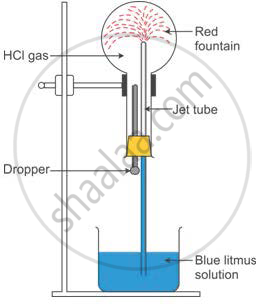
(i) Identify the gas Y.
(ii) What property of gas Y does this experiment demonstrate?
(iii) Name another gas which has the same property and can be demonstrated through this experiment.
The aim of the fountain experiment is to prove that
What property of hydrogen chloride is demonstrated when it is collected by downward delivery (Upward displacement)?
State the following:
The drying agent used in the laboratory preparation of HCl gas.
