Advertisements
Advertisements
Question
Choosing only substances from the list given in the box below, write equations for the reactions which you would use in the laboratory to obtain: Sodium sulphate
| Dilute sulphuric acid | Copper | Copper carbonate |
| Iron | Sodium carbonate | |
| Sodium | ||
| Zinc |
Solution
Sodium sulphate is obtained by the action of dilute sulphuric acid and sodium carbonate.
\[\ce{Na2CO3 + H2SO4_{(dil.)}-> Na2SO4 + H2O + CO2_{(g)}}\]
APPEARS IN
RELATED QUESTIONS
Give balanced chemical equation to prepare the following salt:
Copper chloride using copper carbonate
Write the balanced equation for the preparation of the following salt in the laboratory:
A soluble sulphate by the action of an acid on an insoluble base.
Write the balanced equation for the preparation of the following salt in the laboratory:
An insoluble salt by the action of an acid on another salt.
Write the balanced equation for the preparation of the following salt in the laboratory:
An insoluble base by the action of a soluble base on a soluble salt.
Write the balanced equation for the preparation of the following salt in the laboratory:
A soluble sulphate by the action of an acid on a metal.
(a) What are the terms defined by the following?
(i) A salt containing a metal ion surrounded by other ions or molecules.
(ii) A base which is soluble in water.
(b) Making use only of substances chosen from those given below:
Dilute sulphuric acidSodium Carbonate
Zinc Sodium sulphite
Lead Calcium carbonate
Give equations for the reactions by which you could obtain :
(i) Hydrogen
(ii) Sulphur dioxide
(iii) Carbon dioxide
(iv) Zinc carbonate (two steps required)
Choosing only substances from the list given in the box below, write equations for the reactions which you would use in the laboratory to obtain: Copper sulphate.
| Dilute sulphuric acid | Copper | Copper carbonate |
| Iron | Sodium carbonate | |
| Sodium | ||
| Zinc |
Choosing only substances from the list given in the box below, write equations for the reactions which you would use in the laboratory to obtain: lron(II) sulphate
| Dilute sulphuric acid | Copper | Copper carbonate |
| Iron | Sodium carbonate | |
| Sodium | ||
| Zinc |
Choosing only substances from the list given in the box below, write equations for the reactions which you would use in the laboratory to obtain: Zinc carbonate.
| Dilute sulphuric acid | Copper | Copper carbonate |
| Iron | Sodium carbonate | |
| Sodium | ||
| Zinc |
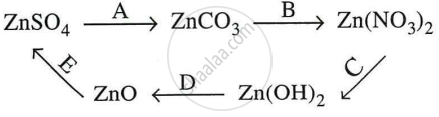
Give equations for the following conversions A to E.