Advertisements
Advertisements
Question
Comment on the nature of two S−O bonds formed in SO2 molecule. Are the two S−O bonds in this molecule equal?
Solution 1
The electronic configuration of S is 1s2 2s2 2p6 3s2 3p4.
During the formation of SO2, one electron from 3p orbital goes to the 3d orbital and S undergoes sp2hybridization. Two of these orbitals form sigma bonds with two oxygen atoms and the third contains a lone pair. p-orbital and d-orbital contain an unpaired electron each. One of these electrons forms pπ- pπ bond with one oxygen atom and the other forms pπ- dπ bond with the other oxygen. This is the reason SO2 has a bent structure. Also, it is a resonance hybrid of structures I and II.

Both S−O bonds are equal in length (143 pm) and have a multiple bond character.
Solution 2
SO2 exists as an angular molecule with OSO bond angle of 119.5°. It a resonance hybrid of two canonical-forms:
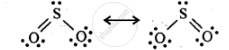
Due to resonance the to π bonds are equal.
APPEARS IN
RELATED QUESTIONS
Explain the structure of sulphur dioxide.
What happens when: SO2 gas is passed through an aqueous solution Fe+3 salt?
How is the presence of SO2 detected?
How is SO2 an air pollutant?
When SO2 gas is passed through the acidified K2Cr2O7 solution, the colour of the solution changes to:
Answer the following question.
Write a chemical reaction to test sulphur dioxide gas. write a chemical equation involved.
Which of the following is not an example of antiseptic drug?
Sulfur dioxide is a ____________.
The number of moles of chlorine required to oxidise 1 mol of sulfur dioxide to sulfur trioxide in presence of water is ____________.
The O-S-O bond angle and hybridization of Sulphur in SO2 molecule is respectively.
When sulphur dioxide combines with chlorine in the presence of charcoal catalyst, the product formed is ______.
The number of lone pair and bond pair of electrons on the sulphur atom in sulphur dioxide molecule are respectively
Write the chemical equation for the preparation of sulphur dioxide from sulphur.
Give reactions to show that SO2 gas acts as a reducing agent.
