Advertisements
Advertisements
Question
Comment upon the following:
rigidity, compressibility, fluidity, filling a gas container, shape, kinetic energy and density.
Solution
- Rigidity: It is the property which helps a substance retain its shape when force is applied to it. Solids are rigid while gases and liquids are not.
- Compressibility: Compressibility is the property that allows particles of matter to be compressed or reduced in volume by applying force or pressure. Gases are very compressible.
- Fluidity: It is the tendency of a substance to flow. Liquids and gases possess fluidity while solids are rigid.
- Filling a gas container: Because of the minimal interparticle force of attraction, gas molecules can move in all directions and fill the container.
- Shape: Solids have a distinct shape, whereas liquids take on the shape of the container in which they are stored, and gases have no shape.
- Kinetic energy: Kinetic energy refers to the energy that particles contain as a result of their motion. Gas particles have the most kinetic energy because they can move freely in all directions. Solids have the lowest kinetic energy because their particles move the least.
- Density: Density refers to the mass of a substance per unit volume. Solids have the highest density because their molecules are tightly packed.
APPEARS IN
RELATED QUESTIONS
The mass per unit volume of a substance is called density.
(density = mass/volume).
Arrange the following in order of increasing density-
air, exhaust from chimneys, honey, water, chalk, cotton and iron.
Give reasons:
We can easily move our hand in air, but to do the same through a solid block of wood, we need a karate expert.
Name two gases which are supplied in compressed form in homes and hospitals.
Why do gases have neither a fixed shape nor a fixed volume?
When an incense stick (agarbatti) is lighted in one corner of a room, its fragrance quickly spreads in the entire room. Name the process involved in this.
Give one example of diffusion of gases in a liquid.
The substance X normally exists in a physical state which can flow easily but does not fill its vessel completely. It also turns anhydrous copper sulphate blue. When substance X is cooled excessively, it changes into a substance Y which has a fixed shape as well as a fixed volume. If, however, the substance X is heated strongly, it changes into a substance Z which has neither a fixed shape nor a fixed volume.
- Name the substances (i) X (ii) Y and (iii) Z.
- What is the process of conversion of X into Y known as ?
- At which temperature X gets converted into Y ?
- What is the process of conversion of X into Z known as ?
- At which temperature X gets converted into Z ?
Explain why?
- A gas fills a vessel completely?
- Camphor disappears without leaving any residue.
Which of the following is TRUE about gaseous state?
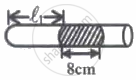 |
|
| Fig. I | Fig. II |
In figure-I, an air column of length ℓ1 is entrapped by a column of Hg of length 8 cm. In figure-II length of same air column at the same temperature is ℓ2. The `ℓ_1/ℓ_2` is:
(1 atm = 76 cm of Hg)