Advertisements
Advertisements
Question
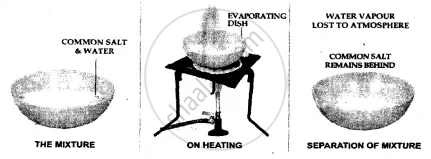
Common salt from a solution of common salt in water
- Give the principle involved in the separation of the mixture
- Give the technique of separation of the mixture.
Solution
By Evaporation
Principle – Based on the evaporation of the liquid component in a soluble solid-liquid mixture.
Evaporation – It is a method used for recovery of the soluble solid from a solution by heating the mixture slowly, in a porcelain crucible on a flame.
For smoother steady heating the porcelain crucible may be kept in a sand bath [a shallow metallic dish filled with sand].
The technique of Separation – The soluble solid can be separated from its liquid component by allowing the liquid component to evaporate either on its own or by heating. The liquid component is lost to the atmosphere The soluble solid component remains behind in the evaporating dish
APPEARS IN
RELATED QUESTIONS
We can separate iron metal from non-magnetic impurities by the method of magnetic separation.
How is distillation method different from evaporation?
Define Evaporation
Draw a neat labelled diagram for the separation of the following mixture.
Naphthalene from sodium chloride
State the technique involved in separating the following:
Potassium chloride from an aqueous solution of potassium chloride.
State the correct technique for the separation of the following mixture.
a liquid component from soluble impurities in the liquid component.
Give a reason for the following statement:
Evaporation of a common salt solution or seawater leaves behind common salt inside the evaporating dish after heating.
Name the process associated with the following
An acetone bottle is left open and the bottle becomes empty.
Give an example of each mixture having the following characteristics. Suggest a suitable method to separate the components of this mixture
A volatile and a non-volatile component.
During summer, Boojho carries water in a transparent plastic bottle to his school. One day he left his bottle in the school. The bottle still had some water left in it. The following day, he observed some water droplets on the inner surface of the empty portion of the bottle. These droplets of water were formed due to
