Advertisements
Advertisements
Question
Pure water from impure water
- Give the principle involved in the separation of the mixture
- Give the technique of separation of the mixture.
Solution
By Distillation
Principle- Based on the distillation of the liquid component in a soluble solid-liquid mixture.
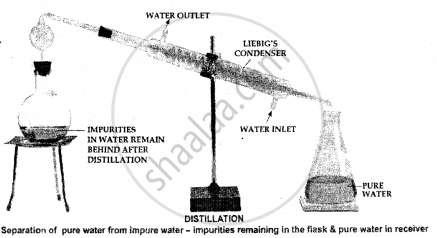
Distillation – It is the process of converting a liquid into vapour by heating in a distillation flask & subsequent condensation of the vapour back into the liquid.
The method is used to separate a liquid from a soluble solid or pure liquid [water] from impure liquid [impure water].
The technique of Separation The soluble solid can be separated from its liquid component or pure water from impure water by placing the mixture i.e. impure water in the distillation flask.
On heating the distillation flask
The solid or solid impurities in the water remain behind in the distillation flask
The liquid or water vaporizes, condenses in the Liebig’s condenser, and is collected in the receiver.
APPEARS IN
RELATED QUESTIONS
How will you separate a mixture containing kerosene and petrol (difference in their boiling points is more than 25°C), which are miscible with each other?
What is the difference between distillation, distillation under reduced pressure and steam distillation ?
On what principle are the following methods of separation based? Give one example of a mixture for each of the methods mentioned in which they are used
Fractional distillation :
Give one word answer
The process by which two miscible liquids are separated
Explain the difference between Separation by distillation and by separating funnel.
State the correct technique for the separation of the following mixture.
a low boiling point liquid from a high boiling point liquid.
Choose the correct option.
Which of the following methods can be used to separate two compounds with different solubilities in the same solvent?
Match the type of mixture of compounds in Column I with the technique of separation/purification given in Column II.
| Column I | Column II |
| (i) Two solids which have different solubilities in a solvent and which do not undergo reaction when dissolved in it. | (a) Steam distillation |
| (ii) Liquid that decomposes at its boiling point | (b) Fractional distillation |
| (iii) Steam volatile liquid | (c) Simple distillation |
| (iv) Two liquids which have boiling points close to each other | (d) Distillation under reduced pressure |
| (v) Two liquids with large difference in boiling points. | (e) Crystallisation |
Assertion (A): Simple distillation can help in separating a mixture of propan-1-ol (boiling point 97°C) and propanone (boiling point 56°C).
Reason (R): Liquids with a difference of more than 20°C in their boiling points can be separated by simple distillation.
A mixture of benzene and chloroform is separated by ______.
