Topics
Some Basic Concepts of Chemistry
- Introduction of Some Basic Concepts of Chemistry
- Nature of Chemistry
- Properties of Matter and Their Measurement
- Laws of Chemical Combination
- Dalton's Atomic Theory
- Atomic and Molecular Masses
- Mole Concept
- Moles and Gases
Introduction to Analytical Chemistry
- Introduction of Analytical Chemistry
- Analysis
- Mathematical Operation and Error Analysis
- Determination of Molecular Formula
- Chemical Reactions and Stoichiometric Calculations
- Limiting Reagent
- Concentration of a Solution
- Use of Graph in Analysis
Basic Analytical Techniques
- Introduction of Some Analytical Techniques
- Purification of Solids
- Crystallisation Method
- Fractional Crystallization
- Simple Distillation Method
- Solvent Extraction
- Chromatography Method
- Adsorption Chromatography
- Partition Chromatography
Structure of Atom
- Subatomic Particles
- Atomic Number and Atomic Mass Number
- Isotopes, Isobars and Isotones
- Drawbacks of Rutherford Atomic Model
- Bohr’s Atomic Model
- Bohr’s Model for Hydrogen Atom
- Quantum Mechanical Model of Atom
Chemical Bonding
- Introduction of Chemical Bonding
- Kossel-lewis Approach to Chemical Bonding - Octet Rule
- Kossel and Lewis Approach to Chemical Bonding
- Kossel-lewis Approach to Chemical Bonding - Formal Charge
- Kossel-lewis Approach to Chemical Bonding - Limitations of the Octet Rule
- Valence Shell Electron Pair Repulsion Theory (VSEPR)
- Valence Bond Theory
- Molecular Orbital Theory
- Parameters of Covalent Bond
- Dipole Moment
- Resonance
Redox Reactions
- Introduction of Redox Reactions
- Oxidation Number
- Balancing Redox Reactions in Terms of Loss and Gain of Electrons
- Redox Reaction and Electrode Potential
Modern Periodic Table
- Introduction of Periodic Table
- Structure of the Modern Periodic Table
- Periodic Table and Electronic Configuration
- Blockwise Characteristics of Elements
- Periodic Trends in Elemental Properties
Elements of Group 1 and 2
- Hydrogen
- Alkali Metals and Alkaline Earth Metals
- Some Important Compounds of Elements of S-block
Elements of Group 13, 14 and 15
- Electronic Configuration of Elements of Groups 13, 14 and 15
- Trends in Atomic and Physical Properties of Elements of Groups 13, 14 and 15
- Chemical Properties of the Elements of the Groups 13,14 and 15
- Catenation
- Allotropy and Allotropes of Carbon
- Molecular Structures of Some Important Compounds of the Group 13, 14 and 15 Elements
- Chemistry of Notable Compounds of Elements of Groups 13, 14 and 15
States of Matter
- Introduction of States of Matter: Gaseous and Liquid States
- Intermolecular Forces
- Characteristic Properties of Gases
- The Gas Laws
- Ideal Gas Equation
- Kinetic Molecular Theory of Gases
- Deviation from Ideal Behaviour
- Liquefaction of Gases and Critical Constant
- Liquid State
Adsorption and Colloids
- Introduction of Adsorption
- Adsorption
- Types of Adsorption
- Factors Affecting Adsorption of Gases on Solids
- Adsorption Isotherms (Freundlich and Langmuir Adsorption Isotherm)
- Applications of Adsorption
- Catalysis
- Adsorption Theory of Heterogeneous Catalysis
- Colloids
Chemical Equilibrium
- Introduction of Chemical Equilibrium
- Equilibrium in Physical Processes
- Equilibrium in Chemical Processes - Dynamic Equilibrium
- Law of Mass Action and Equilibrium Constant
- Homogeneous and Heterogenous Equilibria
- Characteristics of Equilibrium Constant
- Applications of Equilibrium Constants
- Le Chaterlier's Principle and Factors Altering the Composition of Equilibrium
- Industrial Application
Nuclear Chemistry and Radioactivity
- Introduction: Nuclear Chemistry is a Branch of Physical Chemistry
- Classification of Nuclides
- Nuclear Stability
- Radioactivity
- Radioactive Decays
- Modes of Decay
- Nuclear Reactions
- Applications of Radio Isotopes
Basic Principles of Organic Chemistry
- Introduction of Basic Principles of Organic Chemistry
- Structural Representation of Organic Molecules
- Classification of Organic Compounds
- Nomenclature of Organic Compounds
- Isomerism
- Theoretical Basis of Organic Reactions
Hydrocarbons
- Alkanes
- Alkenes
- Alkynes
- Aromatic Hydrocarbons
Chemistry in Everyday Life
- Chemistry in Everyday Life
- Basics of Food Chemistry
- Compounds with Medicinal Properties
- Cleansing Agents
- Distillation Method
- Experiment 1
- Experiment 2
Distillation Method:
Distillation is a process used to separate components of a liquid mixture based on their different boiling points. It involves heating a liquid to form vapour and condensing the vapour back into liquid form. This method is commonly used to separate water from salt solutions and for the purification of impure liquids.
Experiment 1
1. Aim: To separate a mixture of acetone and water using the distillation method.
2. Requirements: Distillation flask, a mixture of acetone and water, thermometer, condenser, etc.
3. Principle: Acetone has a lower boiling point than water, so it vaporises faster. The temperature should not exceed the boiling point of water, which is determined with the help of a thermometer. Acetone will turn into liquid when it passes through the condenser and water will be left behind in the distillation flask. Hence, pure acetone can be obtained.

4. Procedure
- Take the mixture in a distillation flask.
- Fit it with a thermometer and arrange the apparatus as shown
- Heat the mixture slowly, keeping a close watch on the thermometer.
- The acetone vaporises and condenses in the condenser and can be collected from the condenser outlet.
5. Observation: Acetone got vaporised, and while passing through the condenser, it got converted into liquid form again.
6. Inference/Result: Acetone is a volatile liquid and vaporises easily on heating, and water remains in the flask until its boiling point is not received. On cooling, acetone again converts into its liquid form, which is free of water.
Experiment 2
1. Aim: To separate pure water from a salt solution through distillation.
2. Requirements: round-bottom flask, conical flask, saltwater, wire gauze, burner, condenser (tube for condensation), rubber tubing for cooling water supply, tripod stand.
3. Procedure
- Place salt water in the round-bottom flask. Connect the flask to a condenser tube (cooled by circulating water).
- Place the conical flask at the end of the condenser to collect the distilled water.
- Heat the salt water using a burner, with the flask on a wire gauze for stability.
4. Observation
- As the water boils, it forms steam. The steam passes through the condenser, which cools and condenses into water droplets.
- These droplets collect in the conical flask. Once all the water is collected, salt remains in the round-bottom flask.
5. Conclusion: The salt stays behind, and the pure water is collected in the conical flask. Distillation separates soluble substances (like salt) from liquids by evaporating and condensing the liquid. It can also be used to purify impure liquids.
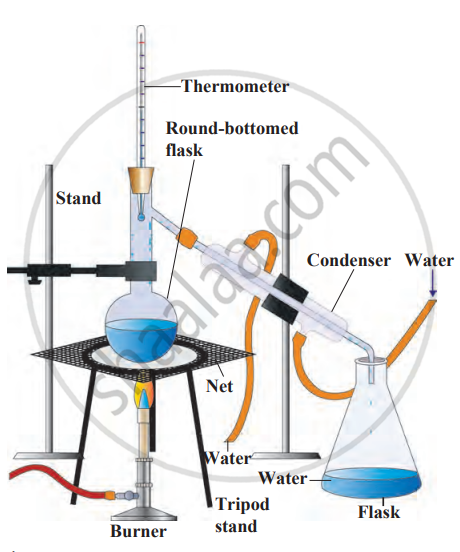
Distillation method
Shaalaa.com | Separate two miscible liquids using distillation
Related QuestionsVIEW ALL [33]
Match the type of mixture of compounds in Column I with the technique of separation/purification given in Column II.
| Column I | Column II |
| (i) Two solids which have different solubilities in a solvent and which do not undergo reaction when dissolved in it. | (a) Steam distillation |
| (ii) Liquid that decomposes at its boiling point | (b) Fractional distillation |
| (iii) Steam volatile liquid | (c) Simple distillation |
| (iv) Two liquids which have boiling points close to each other | (d) Distillation under reduced pressure |
| (v) Two liquids with large difference in boiling points. | (e) Crystallisation |

