Advertisements
Advertisements
Question
Compare all the proposed models of an atom given in this chapter.
Solution
| I. | J.J. Thomson's model of atom | (i) | An atom is a positive-charged sphere with embedded electrons, similar to the seeds of a watermelon. |
| (ii) |
The entire positive charge on the sphere equals the total negative charge on the electrons, indicating that the atom is electrically neutral. |
||
| (iii) | It could not account for the results of Rutherford's scattering tests. | ||
| II. | Rutherford's model of atom | (i) | An atom comprises a small positively charged nucleus at the centre, and electrons orbit it. |
| (ii) | There is a vast vacant area between the nucleus and electrons. | ||
| (iii) | The nucleus contains the vast majority of the atom's mass. | ||
| (iv) | It cannot explain the atom's stability because the spinning electron is accelerated towards the nucleus. Consequently, it will lose energy. Its orbit will become smaller and smaller until the electron falls into the nucleus. | ||
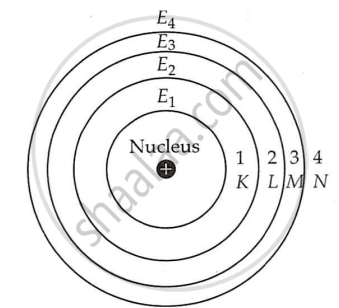
| III. | Bohr's model of atom | (i) | An atom comprises a tiny, positively charged nucleus in the centre and electrons that travel in circular pathways known as orbits. |
| (ii) |
The orbits in which electrons circle in an atom are distinct orbits with set radii and energies. These separate orbits are also known as energy levels or shells. The phrase shell refers to a three-dimensional atom, similar to a ball rather than a plate. These are referred to as stationary states since the energy of the orbits remains constant. These are numbered 1, 2, 3, 4, etc., as we move away from the nucleus, or they are represented by the letters K, L, M, N, etc., as seen in Fig. The energy of these shells grows as one moves away from the nucleus. Using E1, E2, E3, and E4 to represent the energy of the first, second, third, and fourth shells, we get E1 < E2 < E3 < E4. However, as we travel away from the nucleus, the distance between the subsequent energy shells shrinks.
Circular orbits or energy levels or shells around the nucleus |
||
| (iii) | As long as an electron is in a specific orbit, it cannot lose or gain energy. Thus, the atom remains stable and does not collapse. The atom's lowest energy state is known as its ground state. | ||
| (iv) | An electron loses or gains energy only when it jumps from one orbit to another. When energy falls on an electron, it absorbs and moves to an outer shell. The atom is thus considered to be excited. In the excited state, the atom is unstable. It loses or emits energy before returning to a certain internal energy state. In other words, when an electron travels from an inner shell to an outer shell, it absorbs energy, whereas it emits energy when it jumps from an outer shell to an inner. |
APPEARS IN
RELATED QUESTIONS
Draw a sketch of Bohr’s model of an atom with three shells.
Describe Bohr’s model of the atom.
What are the various letters used by Bohr to represent electron shells in an atom?
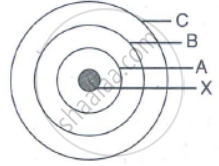
In the figure given alongside
(a) Name the shells denoted by A,B, and C. Which shell has least energy
(b) Name X and state the charge on it
(c) The above sketch is of …………. Model of an atom
Give the postulates of Bohr's atomic model
State in brief the drawbacks of Rutherford's atomic model correlating them with the postulates of Bohr’s atomic model.
State true or false. If false, correct the statement.
Smaller the size of the orbit, lower is the energy of the orbit.
An atom with 3 protons and 4 neutrons will have a valency of
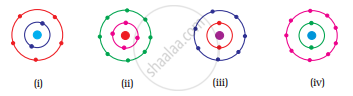
Which of the following in Fig. 4.2 do not represent Bohr’s model of an atom correctly?
Niels Bohr was Born on ______.