Advertisements
Advertisements
Question
Complete the following crossword puzzle (Fig. 3.1) by using the name of the chemical elements. Use the data given in Table.
| Across | Down | ||
| 2 | The element used by Rutherford during his α–scattering experiment | 1 | A white lustrous metal used for making ornaments and which tends to get tarnished black in the presence of moist air |
| 3 | An element which forms rust on exposure to moist air | 4 | Both brass and bronze are alloys of the element |
| 5 | A very reactive non–metal stored under water | 6 | The metal which exists in the liquid state at room temperature |
| 6 | Zinc metal when treated with dilute hydrochloric acid produces a gas of this element which when tested with burning splinter produces a pop sound. | 8 | An element with symbol Pb |

Solution
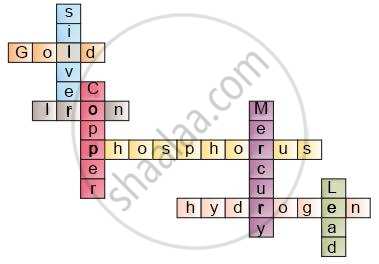
| Across | Down | ||
| 2 | Gold | 1 | Silver |
| 3 | Iron | 4 | Copper |
| 5 | Phosphorus | 6 | Mercury |
| 7 | Hydrogen | 8 | Lead |
APPEARS IN
RELATED QUESTIONS
Write the chemical formula of the Copper nitrate.
Give the formulae of the compounds formed from the following sets of elements :
- calcium and fluorine
- hydrogen and sulphur
- nitrogen and hydrogen
- carbon and chlorine
- sodium and oxygen
- carbon and oxygen
The formula of the nitride of a metal X is XN, state the formula of :
- its sulphate
- its hydroxide.
Write the formula of the following compound:
Sodium bisulphate
Write the formula of the following compound:
Sodium permanganate
Write the formula of the following compound:
Calcium hydroxide
Write the formula of the following compound:
Magnesium bicarbonate
Write the formula of the following compound:
Iron [III] chloride
Write the formula of the following compound:
Iron [III] sulphide
Which of the following represents a correct chemical formula? Name it.
BiPO4
