Topics
The Language of Chemistry
- Matter (Substance)
- Molecules
- Pure Substances
- Elements
- Classification of Molecules
- Symbols Used to Represent Atoms of Different Elements
- Valency
- Variable Valency
- Chemical Formula or Molecular Formula
- Ions (Radicals) and Its Types
- Chemical Formula or Molecular Formula
- Naming Chemical Compounds
- To Calculate the Valency from the Formula
- Chemical Equation
- Balancing Chemical Equation
- Atomic Mass
- Molecular Mass
- Percentage Composition, Empirical and Molecular Formula
- Empirical Formula of a Compound
- Hydrogen
Chemical Changes and Reactions
Water
- Water: Our Lifeline
- Physical Properties of Water
- Chemical Properties of Water
- Water - a Universal Solvent
- Solutions as 'Mixtures' of Solids in Water
- Components of Solutions
- Different Types of Solutions
- Saturated Solutions
- Concentration of a Solution
- Solubility
- Crystals and Crystallisation
- Hydrated and Anhydrous Substances
- Efflorescence, Hygroscopic, and Deliquescence Substances
- Drying and Dehydrous Substances
- Classification of water: Soft and Hard Water
- Causes of Hardness
- Advantage and Disadvantage of Hard Water
- Removal of Hardness of Water
- Salts
- Prevention of Water Pollution
Atomic Structure and Chemical Bonding
- Chemical Bond
- History of Atom
- Dalton’s Atomic Theory
- Elements
- Atoms: Building Blocks of Matter
- Discovery of Charged Particles in Matter
- Electrons (e)
- Protons (p)
- Nucleus
- Neutrons (n)
- J. J. Thomson’s Atomic Model
- Lord Rutherford’s Atomic model
- Neils Bohr’s Model of an Atom
- Structure of an Atom
- Atomic Number (Z), Mass Number (A), and Number of Neutrons (n)
- Atomic Mass
- Electronic Configuration of Atom
- Valency
- Reason for Chemical Activity of an Atom
- Isotopes
- Isobars
- Ionic or Electrovalent Bond
- Ionic or Electrovalent Bond
- The Covalent Bond
- Types of Covalent Bond
- Formation of Covalent Bond
The Periodic Table
- History of Periodic Table: Early Attempts at the Classification of Elements
- Dobereiner’s Triads
- Newland's Law of Octaves
- Mendeleev’s Periodic Table
- Merits and Demerits of Mendeleev’s Periodic Table
- Modern Periodic Law
- The Modern Periodic Table
- Structure of the Modern Periodic Table
- Advantage and Disadvantage of Modern Periodic Table
- Periodic Properties
- Shells (Orbits)
- Valency
- Properties of Elements
- Atomic Radius Or Atomic Size
- Metallic and Non-metallic Characters
- Study of Specific Groups in Periodic Table
- Group I (Alkali Metals)
- Group II (Alkaline Earth Metals)
- Group VIIA Or Group 17 (The Halogens)
- Group Zero or 18 Group (Noble Gases)
- Uses of Periodic Table
- Types of Element: Metals
Study of the First Element - Hydrogen
- Position of Hydrogen in Periodic Table
- Similarities Between Hydrogen and Alkali Metals
- Similarities Between Hydrogen and Halogens
- Hydrogen
- Preparation of Hydrogen
- Application of Activity Series in the Preparation of Hydrogen
- Laboratory Preparation of Hydrogen
- Manufacture of Hydrogen
- Physical Properties of Hydrogen
- Chemical Properties of Hydrogen
- Uses of Hydrogen
- Oxidation, Reduction and Redox Reactions
Study of Gas Laws
- Gases and Its Characteristics
- Molecular Motion : Relationship of Temperature, Pressure and Volume
- The Gas Laws
- Pressure and Volume Relationship or Bolye's Law
- Temperature - Volume Relationship or Charles's Law
- Absolute Zero
- The Temperature and a Thermometer
- Scales of Thermometers
- Gas Equation
- Standard Temperature Pressure (S.T.P.)
- The Effect of Moisture and Pressure
Atmospheric Pollution
- Atmospheric Pollution
- Air Pollution and Its Causes
- Effects of Air Pollution
- Prevention of Air Pollution
- Gaseous Pollutants and Their Effects
- Acid Rain
- Causes of Acid Rain
- Green House Effect
- Advantage of Green House Effect
- Global Warming
- Preventive Measures of Global Warming
- Ozone
- Ozone Layer Depletion
Elements, Compounds and Mixtures
- Differences Between Elements, Compounds, and Mixtures
- Types of Mixtures
- Mixture
- Separation of Mixtures
- Use of Solvent and Filtration
- Concept of Evaporation
- Simple Distillation Method
- Simple Distillation Method
- Chromatography Method
- Centrifugation Method
- Solvent Extraction (Using a Separating Funnel Method)
Matter and Its Composition: Law of Conservation of Mass
- Heat and change of physical state
- Inter-particle Space and Interparticle Attraction and Collision
- Law of Conservation of Mass
Practical Work
- Laboratory Preparation of Hydrogen
- Laboratory Preparation of Oxygen
- Laboratory Preparation of Carbon Dioxide
- Laboratory Preparation of Chlorine
- Laboratory Preparation of Hydrogen Chloride Gas
- Laboratory Preparation of Sulphur Dioxide
- Laboratory Preparation of Hydrogen Sulphide
- Laboratory Preparation of Ammonia Gas
- Laboratory Preparation of Water Vapour
- Laboratory Preparation of Nitrogen Dioxide
- Action of Heat on a Given Substance
- Action of Dilute Sulphuric Acid on a Given Substance
- Dry Test
- Recognition of Substances by Colour
- Recognition of Substances by Odour
- Recognition of Substances by Physical State
- Recognition of Substances by Action of Heat
- Flame Test
- Classification of water: Soft and Hard Water
- Simple Experiments Based on Hard Water and Soft Water
- Water Pollution and Its Causes
- Water Quality
- Strength of Acidic or Basic Solutions
- Prevention of Water Pollution
- Chemical formulae of compounds : A recapitulation
Notes
The chemical formula of a compound is a symbolic representation of its composition. The chemical formulae of different compounds can be written easily. For thisexercise, we need to learn the symbols and combining capacity of the lements.The combining power (or capacity) of an element is known as its valency. Valency can be used to find out how the atoms of an element will combine with the atom(s) of another element to form a chemical compound. The valency of the atom of an element can be thought of as hands or arms of that atom.
Human beings have two arms and an octopus has eight. If one octopus has to catch hold of a few people in such a manner that all the eight arms of the octopus and both arms of all the humans are locked, how many humans do you hink the octopus can hold? Represent the octopus with O and humans with H. Can you write a formula for this combination? Do you get OH4 as the formula? The subscript 4 indicates the number of humans held by the octopus.
FORMULAE OF SIMPLE COMPOUNDS :
The simplest compounds, which are made up of two different elements are called binary compounds. Valencies of some ions are given in Table 3.6. You can use these to write formulae for compounds. While writing the chemical formulae for compounds, we write the constituent elements and their valencies as shown below. Then we must crossover the valencies of the combining atoms.
Examples :
1. Formula of hydrogen chloride
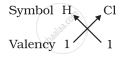
Formula of the compound would be HCl
2. Formula of hydrogen sulphide
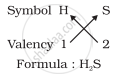
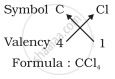
3. Formula of carbon tetrachloride
For magnesium chloride, we write the symbol of cation (Mg2+) first followed by the symbol of anion (Cl- ). Then their charges are criss-crossed to get the formula.
4. Formula of magnesium chloride
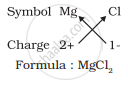
Thus, in magnesium chloride, there are two chloride ions (Cl- ) for each magnesium ion (Mg2+). The positive and negative charges must balance each other and the overall structure must be neutral. Note that in the formula, the charges on the ions are not indicated.
(a) Formula for aluminium oxide:
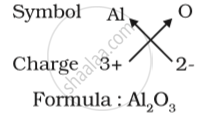
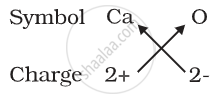
(b) Formula for calcium oxide:
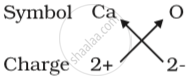
Here the valencies of the two elements are the same. you may arrive at the formula Ca2O2 But we simplify the formula as CaO.
(c) Formula of sodium nitrate:
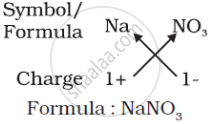
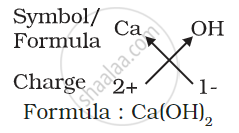
(d) Formula of calcium hydroxide:
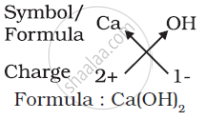
Note that the formula of calcium hydroxide is Ca(OH)2 and not CaOH2. We use brackets when we have two or more of the same ions in the formula. Here, the bracket around OH with a subscript 2 indicates that there are two hydroxyl (OH) groups joined to one calcium atom. In other words, there are two atoms each of oxygen and hydrogen in calcium hydroxide.
Notes
Formation of compounds:

Learn the above table very carefully for the ease of making chemical formulas
1) Formula for calcium oxide:
2) Formula of calcium hydroxide:
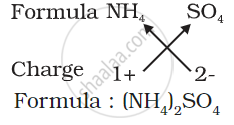
3) Formula of ammonium sulphate:
Note:
-
If the valencies of both elements are the same, they can cancel each other out and are simplified. Eg. It should be CaO and not Ca2O2.
-
In the case of polyatomic ions, the charge is designed to the entire group of ion. Eg. It should be Ca(OH)2 and not CaOH2 as the latter one means 2 atoms of hydrogen only!
