Topics
Chemical Substances - Nature and Behaviour (Chemistry)
Chemical Reactions and Equations
- Chemical Equation
- Balancing Chemical Equation
- Types of Chemical Change or Chemical Reaction
- Direct Combination (or Synthesis) Reaction
- Decomposition Reactions
- Single Displacement Reactions
- Double Displacement Reaction
- Oxidation, Reduction and Redox Reactions
- Corrosion of Metals
- Rancidity of Food and Its Prevention
World of Living (Biology)
Acids, Bases and Salts
- Acids
- Bases (Alkalis)
- Indicators
- Properties of Acids
- Properties of Bases (Alkalis)
- Acid or a Base in a Water Solution
- Similarities and Differences Between Acids and Bases
- Strength of Acidic or Basic Solutions
- Salts
- Important Salts in Daily Life
- Preparation and Uses of Sodium Hydroxide
- Preparation and Uses of Bleaching Powder
- Preparation and Uses of Baking Soda
- Preparation and Uses of Washing Soda
- Preparation and Uses of Plaster of Paris
Metals and Non Metals
- Types of Element: Metals
- Physical Properties of Metals
- Chemical Properties of Metal
- Types of Element: Non-metal
- Physical Properties of Non-metal
- Chemical Properties of Non-metal
- Ionic or Electrovalent Bond
- Reactivity Series of Metals
- Extraction of Reactive Metals
- Refining of Metals
- Corrosion of Metals
- The Covalent Bond
- Prevention of Corrosion
Natural Phenomena (Physics)
Carbon and its Compounds
- Carbon: a Versatile Element
- The Covalent Bond
- Saturated and Unsaturated Carbon Compounds
- Allotropy and Allotropes of Carbon
- Crystalline Allotropes of Carbon: Diamond
- Crystalline Allotropes of Carbon: Graphite
- Crystalline Allotropes of Carbon: Fullerene
- Chains, Branches and Rings of Carbon Compound
- Functional Groups in Carbon Compounds
- Homologous Series of Carbon Compound
- Nomenclature of Organic Compounds
- Properties of Carbon
- Ethanol
- Ethanoic Acid
- Soap
- Detergents
- Cleansing Action of Soap
Effects of Current (Physics)
Life Processes
- Living Organisms and Life Processes
- Nutrients and Nutrition
- Mode of Nutrition in Plant
- Autotrophic Plants
- Heterotrophic Plants
- Different Ways of Taking Food
- Human Digestive System
- The Mouth and Buccal Cavity
- The Teeth and Its Structure
- The Salivary Glands
- Swallowing and Peristalsis
- The Food Pipe/Oesophagus
- The Stomach
- The Small Intestine
- Pancreas
- Absorption of Food
- The Large Intestine
- Assimilation of Food
- Liver
- Respiration
- Respiration
- Breathing in Other Animals
- Osmoregulation
- Types of Respiration: Aerobic and Anaerobic Respiration
- Human Respiratory System
- Circulation in Animals
- Blood
- Composition of Blood: Plasma (The Liquid Portion of Blood)
- Composition of Blood: Red Blood Cells (Erythrocytes)
- Composition of Blood: White Blood Cells (Leukocytes)
- Composition of Blood: Blood Platelets (Thrombocytes)
- Blood Circulatory System in Human
- Human Heart
- Blood Vessels
- Circulation of Blood in the Heart (Functioning of Heart)
- Types of Closed Circulation
- Heart Beat - Heart Sounds "LUBB" and "DUP"
- Function of Platelets - Clotting of Blood (Coagulation)
- Lymph and Lymphatic System
- Blood Pressure (B.P.)
- Transport System in Plants
- Water absorbing organ
- Translocation of Water (Ascent of Sap)
- Transport of Mineral Ions
- Transport of Food
- Transpiration
- Excretion
- Human Excretory System
- Function of the Kidney - “Production of Urine”
- Excretion
Natural Resources
Periodic Classification of Elements
- History of Periodic Table: Early Attempts at the Classification of Elements
- Dobereiner’s Triads
- Newland's Law of Octaves
- Mendeleev’s Periodic Table
- Merits and Demerits of Mendeleev’s Periodic Table
- The Modern Periodic Table
- Periodic Properties
- Valency
- Atomic Radius Or Atomic Size
- Metallic and Non-metallic Characters
Control and Co-ordination
- Control and Co-ordination in Human Being
- Human Nervous System
- Neuron (Or Nerve Cell) and Its Types
- Neuron (Or Nerve Cell) and Its Types
- Nerve Fibres
- Major Division of the Nervous System
- Central Nervous System (CNS)
- Peripheral Nervous System (PNS)
- The Human Brain - Forebrain
- The Human Brain - Forebrain
- Reflex and Reflex Action
- Nervous Pathways in Reflexes
- Reflex Arc
- Coordination in Plant: Tropism in Plants
- Chemical Coordination
- Plant Hormones
- Types of Plant Hormones: Auxins
- Types of Plant Hormones: Gibberellins
- Types of Plant Hormones: Ethylene
- Types of Plant Hormones: Cytokinins
- Types of Plant Hormones: Abscisic Acid (ABA)
- Types of Plant Hormones: Ethylene
- Hormones in Animals
- Human Endocrine System
- Pituitary Gland or Hypophysis Gland
- Thyroid Gland
- Parathyroid Gland
- Pancreas (Islets of Langerhans)
- Adrenal Gland (Suprarenal Gland)
- Reproductive Glands (Gonads)
- Thymus Gland
Internal assessment
How do Organisms Reproduce?
- Accumulation of Variation During Reproduction
- Reproduction
- Mode of Reproduction in Plant
- Asexual Reproduction in Plant
- Natural Vegetative Reproduction
- Sexual Reproduction in Flowering Plants
- Sexual Reproduction in Animals
- Human Reproduction
- The Male Reproductive System
- The Female Reproductive System
- Menstrual Cycle (Ovarian Cycle)
- Reproductive Health
- Sexually Transmitted Diseases (STD)
Heredity
- Accumulation of Variation During Reproduction
- Heredity or Inheritance
- Gregor Johann Mendel – Father of Genetics
- Monohybrid Cross
- Gregor Johann Mendel – Father of Genetics
- Mendelian Inheritance - Mendel’s Law of Heredity
- Sex Determination
- Organic Evolution
- Lamarck’s Theory of Evolution
- Darwinism
- Theories of Origin of Life
- Speciation
- Evolution and Classiffication
- Evidences for Biological Evolution
- Paleobotany
- Evolution by Stages
- Human Evolution
Light - Reflection and Refraction
- Reflection of Light
- Law of Reflection of Light
- Mirrors
- Plane Mirror
- Spherical Mirrors
- Rules for the Construction of Image Formed by a Spherical Mirror
- Images Formed by Spherical Mirrors
- Concave Mirror
- Image Formation by Concave Mirror
- Convex Mirror
- Image Formation by Convex Mirror
- Sign Convention
- Mirror Equation/Formula
- Linear Magnification (M) Due to Spherical Mirrors
- Introduction to Refraction of Light
- Refraction of Light Through a Rectangular Glass Slab
- Refractive Index
- Spherical Lens
- Images Formed by Sperical Lenses
- Guideline for Image Formation Due to Refraction Through a Convex and Concave Lens
- Concave Lens
- Images Formed by Concave Lenses
- Convex Lens
- Images Formed by Convex Lenses
- Sign Convention
- Lens Formula
- Magnification Due to Spherical Lenses
- Power of a Lens
The Human Eye and the Colourful World
- Human Eye
- Working of the Human Eye
- Eye Defect and Its Correction: Myopia Or Near-sightedness
- Eye Defect and its Correction: Hypermetropia or Far-sightedness
- Eye Defect and Its Correction: Presbyopia
- Care of the Eyes
- Refraction of Light Through a Prism
- Prism
- Dispersion of Light Through Prism and Formation of Spectrum
- Atmospheric Refraction
- Application of Atmospheric Refraction
- Scattering of Light and Its Types
- Applications of Scattering of Light
Electricity
- Electricity
- Electric Current
- Electric Circuit
- Potential and Potential Difference
- Symbols and Functions of Various Components of an Electric Circuits
- Ohm's Law (V = IR)
- Factors Affecting the Resistance of a Conductor
- Electrical Resistivity and Electrical Conductivity
- Resistors in Series
- Resistors in Parallel
- Effects of Electric Current
- Heating Effect of Electric Current
- Electrical Power
Magnetic Effects of Electric Current
- Magnetic Effect of Electric Current
- Magnetic Field
- Properties of magnetic lines of force
- Magnetic Field Due to a Current Carrying Straight Conductor
- Right-hand Thumb Rule
- Magnetic Field Due to Current in a Loop (Or Circular Coil)
- Magnetic Field Due to a Current Carving Cylindrical Coil (or Solenoid)
- Force on a Current Carrying Conductor in a Magnetic Field
- Electric Motor
- Electromagnetic Induction
- Faraday's Laws of Electromagnetic Induction
- Electric Generator
- Alternating Current (A.C.) Generator
- Direct Current Motor
- Household Electrical Circuits
- Distinction Between an A.C. Generator and D.C. Motor
- Types of Current
Our Environment
Sources of Energy
- Source of Energy
- Conventional energy resources or non-renewable energy resources
- Fossil Fuels
- Heat Energy (Thermal Energy)
- Hydroelectric Energy
- Bio-energy
- Wind Energy
- Solar Energy
- Solar Energy Devices
- Energy from the Sea
- Geothermal Energy
- Nuclear Energy
- Nuclear Fission
- Forms of Energy
- Environmental Consequences
- How Long Will an Energy Source Last Us?
Sustainable Management of Natural Resources
- Sustainability of Natural Resources
- Case Study: Ganga Pollution and Ganga Action Plan
- Solid Waste Management
- Five R’s of Waste Management
- Protecting our environment
- Forests: Our Lifeline
- Stakeholders of Forest
- Conservation of Forest
- Conservation of Wildlife
- Water Management (Conservation of Water)
- Fresh Water Management
- Non-crystalline/Amorphous Forms: Coal
- Petroleum
- Conservation of Coal, Petroleum, and Natural Resources
- Overview of Natural Resource Management
- Ionic bond or Electrovalent bond
- Mechanism of formation of ionic bond
- Lattice enthalpy
- Factors affecting the formation of an ionic bond
i) Low ionization enthalpy
ii) High negative electron gain enthalpy
iii) Large lattice enthalpy - Characteristics of ionic or electrovalent compounds
- Difference between ionic bond and covalent bond
- Covalent character in ionic bond
- Fajan's rules
Introduction of Ionic Bond:
An ionic bond is a type of chemical bond formed through the transfer of electrons from one atom to another. It typically occurs between a metal and a nonmetal.
- In this process, the metal atom loses one or more electrons to become a positively charged ion (cation), while the nonmetal gains these electrons to become a negatively charged ion (anion).
- The electrostatic force of attraction between the oppositely charged ions holds them together in a stable structure, forming an ionic bond.
- Ionic bonds are responsible for the formation of ionic compounds, such as sodium chloride (NaCl).
- These compounds exhibit unique properties, including high melting and boiling points, solubility in water, and the ability to conduct electricity in molten or aqueous states.
- The ionic bond allows atoms to achieve a stable electron configuration, often following the octet rule, which makes them energetically stable.
Formation of Ionic Bonds
(a) Formation of Ionic Bond in NaCl (Sodium Chloride):
- Sodium (Na): Atomic number 11, configuration 2,8,1. It has 1 valence electron in its outermost shell (M shell).
- Chlorine (Cl): Atomic number 17, configuration 2,8,7. It has 7 valence electrons and needs 1 more electron to complete its octet.
- Sodium loses 1 electron, becoming a Na⁺ cation (positively charged ion). New configuration of Na⁺: 2,8 (stable octet).
- Chlorine gains the electron lost by sodium, becoming a Cl⁻ anion (negatively charged ion). New configuration of Cl⁻: 2, 8, 8 (stable octet).
- The oppositely charged ions (Na⁺ and Cl⁻) are held together by the electrostatic force of attraction, forming an ionic bond.
- The compound formed is sodium chloride (NaCl).
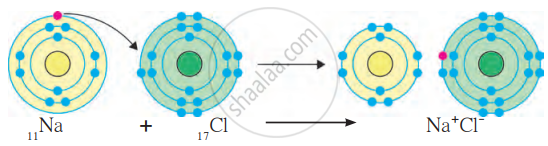
Formation of Ionic bond of NaCl
(b) Formation of Ionic Bond in MgCl₂ (Magnesium Chloride):
- Magnesium (Mg): Atomic number = 12, electronic configuration = 2, 8, 2.
- Magnesium has 2 valence electrons in its outermost shell. It needs to lose 2 electrons to achieve a stable octet configuration.
- Chlorine (Cl): Atomic number = 17, electronic configuration = 2, 8, 7.
- Chlorine has 7 valence electrons in its outermost shell. Each chlorine atom needs 1 electron to complete its octet.
- Magnesium loses 2 electrons, forming a Mg²⁺ cation with a stable configuration of 2, 8.
- Each chlorine atom gains 1 electron from magnesium to form a Cl⁻ anion with a stable configuration of 2, 8, 8.
Mg → Mg²⁺ + 2e⁻
Cl + e⁻ → Cl⁻
Magnesium loses 2 electrons, and these are gained by 2 chlorine atoms.
Mg²⁺ + 2Cl⁻ → MgCl₂
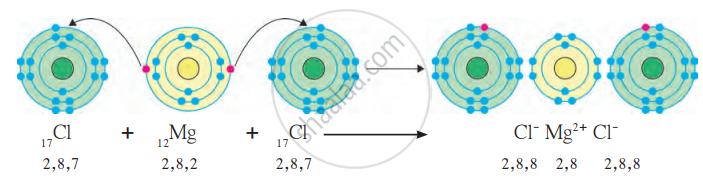
Formation of Ionic bond in MgCl₂ molecule
(c) Formation of Ionic Bond in KF (Potassium Fluoride):
- Potassium (K): Atomic number = 19, electronic configuration = 2, 8, 8, 1.
- Potassium has 1 valence electron in its outermost shell. And it needs to lose 1 electron to achieve a stable octet configuration.
- Fluorine (F): Atomic number = 9, electronic configuration = 2, 7.
- Fluorine has 7 valence electrons in its outermost shell. And it needs to gain 1 electron to complete its octet.
- Potassium loses 1 electron, forming a K⁺ cation with a stable configuration of 2, 8, 8.
- Fluorine gains 1 electron from potassium to form a F⁻ anion with a stable configuration of 2, 8.
K → K⁺ + e⁻
F + e⁻ → F⁻
Potassium loses 1 electron, and fluorine gains 1 electron, ensuring charge balance:
K⁺ + F⁻ → KF

Formation of Ionic bond in KF molecule
(d) Formation of Ionic Bond in CaO (Calcium Oxide):
- Calcium (Ca): Atomic number = 20, electronic configuration = 2, 8, 8, 2.
- Calcium has 2 valence electrons in its outermost shell. And it needs to lose 2 electrons to achieve a stable octet configuration.
- Oxygen (O): Atomic number = 8, electronic configuration = 2, 6.
- Oxygen has 6 valence electrons in its outermost shell. And it needs to gain 2 electrons to complete its octet.
- Calcium loses 2 electrons, forming a Ca²⁺ cation with a stable configuration of 2, 8, 8.
- Oxygen gains 2 electrons from calcium to form an O²⁻ anion with a stable configuration of 2, 8.
Ca → Ca²⁺ + 2e⁻
O + 2e⁻ → O²⁻
Calcium loses 2 electrons, and oxygen gains 2 electrons, ensuring charge balance:

Formation of Ionic bond in CaO molecule
Properties of Ionic Compounds
Melting point and boiling point of some ionic compounds:
| Ionic Compound | Melting Point (K) | Boiling Point (K) |
|---|---|---|
| NaCl | 1074 | 1686 |
| LiCl | 887 | 1600 |
| CaCl₂ | 1045 | 1900 |
| CaO | 2850 | 3120 |
| MgCl₂ | 981 | 1685 |
- Ionic compounds are solid and hard due to the strong electrostatic force between positive and negative ions but are generally brittle, breaking when pressure is applied.
- They have high melting and boiling points because a large amount of energy is needed to overcome the strong ionic bonds.
- Ionic compounds are typically soluble in water, as water's polarity helps separate the ions, but they are insoluble in non-polar solvents like kerosene or petrol.
- In solid form, ionic compounds do not conduct electricity because the ions are fixed in place in the crystal lattice and cannot move.
- In molten form or aqueous solutions, ionic compounds conduct electricity as the ions are free to move and carry electric charge.
- Ionic compounds are characterised by a rigid lattice structure, which provides stability but limits flexibility.
