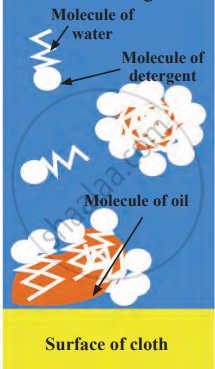
1. Aim: To demonstrate how detergent helps mix oil and water, forming a uniform mixture.
2. Requirements: a clean glass bottle, water, oil (any cooking oil), and detergent solution.
3. Procedure
- Take some water in a clean glass bottle.
- Add a small amount of oil to the water.
- Observe that the oil forms a separate layer on top of the water (oil floats).
- Shake the bottle vigorously and let it settle. You will notice that the oil floats back on the water again.
- Now add a few drops of detergent solution to the same mixture.
- Shake the bottle again vigorously.
- Observe that the mixture becomes uniform (homogeneous) and looks milky.

4. Conclusion: Without detergent, oil and water remain separate because oil is lighter and does not mix with water. When detergent is added, it breaks down the oil into tiny droplets, allowing it to mix with water. This shows that detergent helps oil and water form a stable mixture by acting as a bridge between them.